GC³ Technical Manual: Corrosion
Corrrosion
Introduction
Corrosion of metals in cooling and boiler water systems occurs in
differing forms depending on the condition of the water, temperature,
flow rate, etc. This chapter discusses types of corrosion, the causes
and mechanisms involved, and methods used to inhibit corrosion.
After reading this chapter, you should have a firm grasp of the mechanical
aspects of corrosion, along with a basic understanding of corrosion
inhibition in water systems. Keep in mind that all of the information
contained in this section will prove useful in designing a chemical
approach to corrosion inhibition.

The Electrochemical Nature of Corrosion
Corrosion is the natural process of deterioration of metals and alloys
in a corrosive environment. This is a very broad definition, but
corrosion occurs in a wide variety of forms, both in pure metals and
in alloys. This discussion considers primarily the two most frequently
occurring forms of corrosion, general corrosion and pitting. General
corrosion is the wasting away of a metal or alloy in a corrosive environment,
resulting in an actual decrease in the thickness or size of the original
metallic structure. This wasting away occurs relatively uniformly over
the surface exposed to the corrosive environment. Pitting is a form of
localized corrosion in which a small portion of the metallic structure
is corroded at a rate much faster than the bulk of the structure.
Metals such as steel and copper and alloys such as brass and stainless steel
appear to be fairly rugged and able to withstand a great deal of physical
abuse. This is not true when these metals are surrounded by a corrosive
environment. They can be quickly reduced to thin, rusty or oxide-encrusted
specimens. To put it another way, these metals always have a tendency
to return to their naturally occurring forms.
Metallic elements such as iron, copper, zinc and nickel occur naturally
in the form of oxides, sulfides and carbonates. In metal making, this
natural process is reversed and the metallic element is separated from
its oxide. This requires a great deal of energy, as anyone who has seen
a blast furnace can tell you. The resulting metal or alloy is in a
high-energy state and, under the right conditions, it will attempt to
return to its more natural, lower-energy, reacted state. A detailed
corrosion study of a piece of metal is the study of how this happens,
the rate at which it happens and what causes it to happen.
There are several conditions that must be met before these reactions can occur.
-
The metal, in this case, iron, must be reactive. It must be inherently
unstable in the metallic form, thereby tending to corrode.
-
The metal must be in contact with an electrolyte. An electrolyte is
a solution, usually aqueous, which can conduct electric current and
support ionized species.
-
The electrolyte must contain dissolved species. This can be either
dissolved gases,such as oxygen or chlorine, or dissolved ions, such
as the hydrogen ion, which acts as an oxidizing agent.
-
The kinetics of the situation (the rate at which the corrosion
reactions can occur)must be rapid enough to be of practical
significance.
The first requirement, that the metal must have sufficient reactivity,
is exhibited by metals such as iron, copper and steel. They readily
corrode under the proper conditions. On the other hand, gold and platinum
are more noble metals and do not react readily with their environment.
Without the presence of dissolved gases or minerals in an electrolyte,
such as water, even highly reactive metals do not corrode. Water contains
many types of minerals.
Electrode Potentials and the Galvanic Series
Corrosion reactions are a combination of oxidation and reduction reactions.
Oxidation is the electrochemical process by which an element or species
looses electrons and increases its valence state. A metal transforming
to a metal ion with the simultaneous loss of an electron is an example.
M <--> M+ + e-
Reduction is the electrochemical process by which an element or species
acquires one or more electrons. Thus reducing its valence. The
transformation of hydrogen ions to atomic hydrogen is an example.
H+ + e- <--> H0
When reactions of these types occur, they never occur in isolation, only
in pairs or combination. In fact, the oxidation process, which produces
more electrons, depends on the simultaneous consumption of those electrons
by a reduction reaction. If no reduction reaction is available, no
oxidation occurs. In these cases, the species which undergoes a reduction
reaction is called the oxidizing agent.
The quantitative study of oxidation/reduction reactions has resulted in
two useful concepts:
-
Oxidation - reduction potentials (which apply to elements and
compound)
-
Galvanic series (which applies to alloys in their environments)
Oxidation-Reduction Potentials
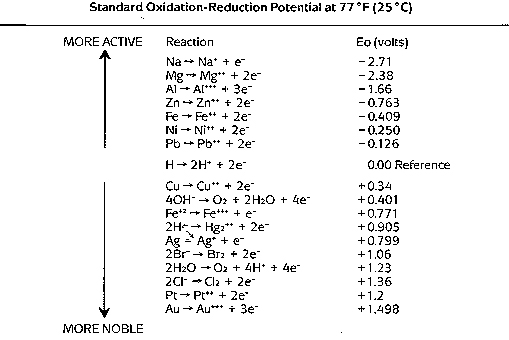
The Table below is a listing of some useful oxidation-reduction potentials.
These values represent the thermodynamic tendency for the indicated reaction
to occur on a relative basis. All potential values are compared to an
arbitrary value of 0.00 volts which is assigned to the hydrogen oxidation
reaction. The more negative a value, the more likely the reaction will
proceed in the direction shown in the Table. Thus we see that zinc
oxidation:
Zn <--> Zn+2 + 2e- E = -0.763 volts
is more likely to occur than iron oxidation
Fe <--> Fe+2 + 2e- E = -0.44 volts
which, in turn, is more likely than hydrogen oxidation
H2 <--> 2H+ + 2e- E = 0.00 volts
Some other generalizations drawn from the standard oxidation reduction
potential table are:
-
Oxygen is a stronger oxidizing agent than hydrogen ion.
-
Iron is more reactive than lead, copper or silver.
-
Gold is very unreactive.
The Galvanic Series
The following Table is a simple version of the galvanic series of alloys
in seawater. Because electrode (oxidation/reduction) potentials only apply
to pure elements and true compounds, another system was developed to compare
the relative reactivity of alloys in an environment. This series has the
added advantage of allowing you to predict the galvanic behavior of certain
alloy pairs in an environment.
If a pair of alloys listed in the series are coupled, the alloy higher in
the list will be corroded more rapidly than if it were uncoupled, and the
alloy lower in the series will be protected, or corrode more slowly than if
it were uncoupled. The table shows why alloys of aluminum and magnesium are
galvanically coupled to steel to protect the steel. Coupling steel to copper,
brass or stainless steel accelerates the corrosion of steel.
Corrosion Reactions and Corrosion Mechanisms
Nearly all corrosion reactions of practical interest are oxidation-reduction
reactions. The oxidation portion of the reaction results in the actual
metal loss, while the reduction portion of the reaction drives the whole
process. The most common corrosion reaction is the rusting of steel in
water:
4Fe + 6H2O + 302 <--> 4Fe(OH)3
In neutral or alkaline solution the individual reactions are:
Fe <--> Fe+2 + 2e- Step one (OX)
Fe+2<--> Fe+3 + e- Step two (OX)
Fe <--> Fe+3 + 3e- Overall reaction
O2 + 2H20 + 4e- <--> 4OH- Reduction reaction
The mechanism of this reaction is complex. The oxygen attacks the surface
of the iron and oxidizes it, releasing ferrous and hydroxyl ions into the
surrounding water. A secondary reaction then takes place, further oxidizing
the ferrous ion to the ferric ion. This species forms an insoluble
precipitate of iron hydroxide that tends to stick onto the iron surface.
The initial stages of the reaction occur quite rapidly. The oxygen has free
access to the surface, where it diffuses and reacts. As ferrous and
hydroxide ions build up near the surface, the oxygen near the surface
becomes depleted, and the reactions begin to slow down. As the reactions
proceed, ferric hydroxide forms a gel near the surface and further restricts
oxygen diffusion to the surface. This is commonly observed in the form of
barnacles. Eventually the reaction slows to an equilibrium rate governed
by the rate at which oxygen diffuses through the rust. Over a period of
time, the corrosion rate of the iron gradually decreases and levels out.
Common Corrosive Agents
Most, but not all, of the common corrosive agents encountered in industrial
waters are gases. The reactions of importance are:
2H+ + 2e- <--> 2H <--> H2
O2 + 4H+ + 4e- <--> 2H2O
O2 + 2H2O + 4e- <--> 4OH-
HCO3- + e- <--> H0 + CO3-2
Cl2 + 2e- <--> 2Cl-
Other corrosive agents occur in industrial waters, although infrequently.
Among these are the ferric ion (Fe+3), sulfide ion (S-2), bromine (Br2)
and cupric ion (Cu+2).
Corrosion Product Films
All corrosion reactions produce corrosion by-products. In some environments,
these products are very soluble and no films actually form on the surface
of the corroding metal. A corrosion process characterized by the absence of
a tenacious film is called active corrosion. In other media, corrosion
products form insoluble hydroxides, carbonates, oxides and sulfates. Some
are loose and porous, allowing diffusion to and from the metal surface.
These types of films do not protect surfaces from further corrosion.
A few films which form as a result of corrosion are very adherent, tight,
and nonporous. These are considerably more protective toward further
corrosion, primarily because they limit access of corrosives to the metal
surface.
Some alloys are inherently unreactive. Aluminum and stainless steels
form corrosion product films of mixed oxides that are so non-reactive and
that completely protect the base metal after a short period of active
corrosion. These alloys are passive, and the films are called passive films.
Types of Corrosion
General Corrosion
General corrosion is the most common type of corrosion. It is defined as
the uniform loss of metal from the entire exposed surface of the metal.
Pitting Corrosion
Pitting is a type of localized corrosion in which a small portion of the
exposed surface experiences very high corrosion rates resulting in small
holes in the metal surface. This type of corrosion usually occurs when
general corrosion rates are low. Pitting is especially dangerous because
the metal loss rates can be very high. The result is unexpected failures in
pipes and tubes.
Crevice Corrosion
Crevice corrosion is another form of localized corrosion. It occurs in
crevices on metal structures. Corrosion materials build up in the space of
the crevice and create a highly localized and very corrosive environment.
Certain anions, such as chlorides, promote the hydrolysis reactions that
cause the problem.
Underdeposit Corrosion
This is a special type of crevice corrosion where the crevice or space is
caused by a deposit on the metal surface. Scale, corrosion products or a
variety of other debris can cause deposits under which accelerated corrosion
occurs. After deposits are formed, it is difficult to stop underdeposit
corrosion, because the deposits make it difficult to get corrosion inhibitors
to the metal surface suffering the high corrosion rates.Another form of
underdeposit corrosion common in open recirculating cooling systems is caused
by the attachment of biomasses to metal surfaces. The biomass produces by-
products that are corrosive to most metals and are held next to the metal
surface by the biomass.
Galvanic Corrosion
When dissimilar metals are connected in an electrolytic solution under the
proper conditions, one metal will experience accelerated corrosion. The
alloy highest in the galvanic series will corrode faster. The relative
areas of the two alloys are important. If the area of the more active alloy
is small compared to the area of the noble metal, then the severity of the
galvanic attack will be greater.
Impingement
Impingement is an accelerated form of corrosion that occurs when a metal
surface, covered by a protective film, is damaged by mechanical or hydraulic
wear or abrasion. Mechanical abrasion will remove protective films, but the
effect of high fluid velocity, intense turbulence and cavitation can
accelerate this process. These effects are most often observed at inlets to
heat exchanger tubes, at piping elbows, in piping downstream of pumps, and
on pump impellers. Copper and copper alloys are especially sensitive to
impingement.
Operational Factors Affecting Corrosion Rates
Temperature
As a general rule, increasing temperature increases corrosion rates. This
is due to a combination of factors- first, the common effect of temperature
on the reaction kinetics themselves and the higher diffusion rate of many
corrosive by-products at increased temperatures. This latter action delivers
these by-products to the surface more efficiently.
Occasionally, the corrosion rates in a system will decrease with increasing
temperature. This can occur because of certain solubility considerations.
Many gases have lower solubility in open systems at higher temperatures.
As temperatures increase, the resulting decrease in solubility of the gas
causes corrosion rates to go down.
pH
Corrosion rates almost always increase with decreasing pH (increasing
acidity). This is a direct result of increasing the concentration of
an aggressive ion (H+) and increasing the solubility of most potentially
corrosive products.
Oxygen Concentration
Oxygen's role in corrosion is as an aggressive gas or oxidizing agent.
As its concentration increases, corrosion rates increase until the rates
of diffusion to the surfaces reach a maximum. The same principles apply
to most other oxidizing agents, such as Cl2, H+, Br2.
Fluid Velocity
The dependence of corrosion rate on fluid velocity is complex. In general,
the higher the velocity, the higher the corrosion rate. At very low
velocities, even zero, there are diffusion effects that can cause corrosion.
As fluid velocities increase from stagnant to moderate values, the corrosion
rates increase. Then, as the limit of diffusion at a particular temperature
is reached, further increases in velocity have little effect on the corrosion
rate. At some point, however, the velocity reaches such high values that
the surface film of the metal begins to be damaged. At these velocities,
the corrosion rates resume increasing with the higher velocities.
Suspended Solids
An increase in suspended solids levels will accelerate corrosion rates.
These solids include any inorganic or organic contaminants present in the
water. Examples of these contaminants include clay, sand, silt or biomass.
Corrosion Inhibitors
To inhibit corrosion, small amounts of corrosion inhibitors can be added
to water systems and process streams to reduce corrosion rates to acceptable
values. In general, corrosion inhibitors incorporate themselves into
corrosion product films in such a way as to increase the film's capacity
to prevent corrosion. The process of corrosion inhibition is related to the
metal surface and the processes occurring in waters on that surface. The
polar nature of some molecules promotes adsorption, but the idea that
corrosion inhibitor films act as barriers is erroneous. The adsorption of
these molecules is accompanied by the companion process of desorption. An
inhibitor molecule usually is in constant motion, being adsorbed and
desorbed between the fluid and the corrosion product film. The rate of
adsorption onto the surface is dependent on the nature of the molecule, as
well as the concentration of the inhibitor in the fluid. The same is true
for the process of desorption. It is important in inhibitor treatment to
maintain a sufficient concentration of the molecule in the fluid so that the
adsorption rate at least equals the desorption rate. This process is
commonly referred to as passivation.
Types of Corrosion Inhibitors
Organic
These materials are characterized by high molecular weight structures,
incorporating nitrogen or phosphorous groups. They are usually highly polar
molecules.
Phosphate Esters
Phosphonates
Inorganic
Salts of some metals and amphoteric elements act as corrosion inhibitors.
Quite often these materials have tenacious film-forming or passivation
effects. In some instances, they react with the metal surface.
Chromate Salts
Zinc Salts
Molybdate Compounds
Phosphates
Nitrite Salts
Silicate Compounds
Corrosion in Recirculating Cooling Systems
A recirculating cooling system is a perfect environment for corrosion. All
of the conditions for producing significant levels of corrosion are present.
Reactive metals, in contact with electrolyte-containing corrosive elements,
accelerate the reactions. Recirculating systems have several complicating
factors likely to aggravate the corrosion problems even further:
-
Heat Transfer: Heat exchanger surfaces corrode more rapidly than other
metal surfaces in the system.
-
Fouling and Scale Formation: Not only can fouling cause underdeposit
corrosion problems, but it may frequently hinder corrosion control
procedures by shielding the metal surface from access to chemical
corrosion inhibitors.
-
Biofouling: Biomasses can form on metal surfaces. Accelerated corrosion
can occur underneath these biomasses due to the operation of corrosive
by-products resulting from metabolic processes. The mass itself can
hinder the action of detergent materials or chemical corrosion inhibitors
by presenting a physical barrier between those materials and the metal
surfaces requiring protection.
-
Physical Process Conditions: High fluid velocity, very low fluid
velocity, excessive turbulence and similar physical process
characteristics may have a strong influence on the corrosion problems
in a system. Very high fluid velocity in some condenser tubes may cause
a mildly corrosive water to inflict severe damage on copper alloy tubes.
Low flow rates, especially if they result in dead areas, prevent the
efficient distribution of chemicals such as chlorine, corrosion inhibitor
or scale preventative.
-
Control Upsets: Most cooling water towers are partly dependent on
parameters that are frequently under automatic or semiautomatic control.
If that control fails, then the corrosion rates can increase drastically.
For example, if acid addition to adjust pH is out of control, the result
if very high corrosion rates. A wide variety of upsets will directly or
indirectly effect corrosion rates.
-
Process Leaks: If a leak develops in a cooling water system, the results
can be disastrous. Corrosion rates can increase at dramatic rates. Leaks
must be detected and stopped as quickly as possible.
Solutions to Corrosion Problems in Water Systems
Economic considerations are normally the primary influence on decisions that
are made when choosing a corrosion control program. The economic benefits
of controlling corrosion in process water systems include savings on
equipment losses, savings in production downtime from unexpected corrosion
failures, energy savings on heat transfer surfaces, and savings on the
treatment of aqueous plant effluents. One way to solve corrosion problems
in industrial water systems (seldom selected because of its high cost) is
the engineering solution. This might come in the form of substituting
corrosion-resistant alloys for mild steel in areas that are subject to
corrosion. Sometimes coatings of various types are used to protect steel
from potentially corrosive waters. A more practical solution involves the
increasing use of nonmetallic materials, such as plastics, in water handling
systems.
Another method of solving corrosion problems is to make operational changes.
For example, the exclusion of a corrosion stream into the water system by
segregating it may solve a problem, or at least isolate it to a part of the
plant where it may be economically handled. Many times, however, this is not
a feasible solution.
But by far the most widely used form of corrosion control in industrial
water systems is a combination of control and the use of specialty chemical
corrosion inhibitors. For many years this was accomplished in cooling
systems through the use of a simple, low-cost inhibitor package whose major
active ingredient was sodium chromate. These treatments had the capability
to effectively control corrosion (producing corrosion rates of 1 mpy or less)
while at the same time reducing microbiological growth and eliminating scale
formation on heat transfer surfaces. Legislation has reduced the amount of
heavy metals allowed in many plant effluents, making it necessary to
substitute non-heavy metal inhibitors (organics) for those used in the past.
Often the transition requires control changes as well as a change in
chemical treatment. Whereas the heavy metal programs performed well near
neutral pH and required few additional biocides, the new programs require
stable operating conditions at a higher pH, which means the addition of a
scale inhibitor or dispersant, as well as a biocide for bacterial
control.
Because the modern programs depend heavily on good control. it is necessary
to monitor the process conditions of importance and monitor the performance
of the chemical additives in the system on a frequent, if not continuous,
basis.
Most cooling water systems have a general operating envelope within which
the system must operate. This is usually determined by a combination of
factors such as the quality and quantity of source water available, the
cost of water disposal, the heat load, and others. A program of corrosion
control (in combination with a scale control program) is set up based on
operational targets of total dissolved solids content, pH, recirculation
rate, etc. This means that both operational variables and the corrosion
rates need to be monitored.
What Should a Corrosion Control and Monitoring
Program Contain?
In order to effectively put together a corrosion control program, the
specific corrosion problem or problems involved must be identified by the
type of corrosion (general or localized) and the causes, if known. Once the
symptoms and problems have been identified, it will be possible to select
a method of control and a method of monitoring the various parameters to
confirm that corrosion is under control, Analysis of the data by operations
personnel is also a necessary part of any program. These data must be used
to help maintain expected corrosion rates over a long period of time.

