GC³ Technical Manual: Deposition
GC³ Technical Manual: Deposition
Introduction
Water is one of the Earth's most unusual features.
It is continuallydissolving or depositing solids. The phrase "depositing solids" encompasses
the scope of this chapter. These deposited solids are classified as scale
or foulants. This chapter identifies the important types of scale and
foulants, examines how the solids are deposited (mechanisms influencing
deposition) and recommends treatment of these scaling/fouling waters.
Having read this chapter, you should know:
1. The difference between scaling and fouling
2. Mechanisms and factors involved in scaling and fouling
3. Types of scales and foulants commonly found in cooling water applications
4. How to predict the scaling tendency of a water sample
5. Methods used to control scale
6. Basic scale inhibitor and dispersant chemistries

Scale Deposition Overview
The causes of scale and other fouling deposits are many, varied and
extremely complex. Investigators have studied scale and deposit mechanisms
in an effort to understand them, quantify them, and develop remedial or
preventative treatments. Deposits in cooling waters are generally classified
either as scale or as foulants, even though these deposits commonly occur
together. Scale is a hard, adherent mineral deposit that usually
precipitates from solution and grows in place. It is a crystalline
form of deposit. Foulants, on the other hand, are usually high in organics.
More often than not, they are formed from suspended solids at a point in the
system other than where they deposit. Foulants tend to be amorphous or
noncrystalline.
Cooling waters contain a large number of these potential scale- and
deposit-causing constituents. These include soluble ions (such as calcium,
magnesium, soluble silica,zinc and iron salts) that precipitate as insoluble
deposits when they encounter changes in water temperature, pH, concentration
or incompatible additives. Examples of deposits are calcium carbonate,
calcium phosphate, silica, iron hydroxides, sulfides, calcium sulfate,
magnesium salts, zinc phosphate and zinc hydroxide.
Fouling deposits can occur from natural or artificial causes. Although
these deposits are generally less adherent than mineral scale, they cause
similar problems. Examples of some of the more common natural foulants are
sand, mud, silt, clay, natural organics, dust and debris. Corrosion
by-products, process contaminants, pretreatment carryover and incompatible
chemical treatments are a few examples of artificially induced foulants.
Microbiological growth such as algae and bacteria are some of the most
common organic foulants in cooling water systems.
Scale Deposition - Mechanisms and Influencing Factors
Scale deposition, unlike other types of deposition, is a complex
crystallization process. The time it takes for an initial scale layer to
form and its subsequent rate of growth are determined by the interaction of
several rate processes (e.g., supersaturation, nucleation, diffusion,
chemical reaction and molecular arrangement of the scale crystal lattices).
Most, though not all, mineral scale-forming constituents are inversely
soluble (i.e., their solubility decreases as water temperature increases).
Therefore, when these supersaturated solutions contact heat transfer
surfaces, they precipitate solids due to their lower equilibrium solubility.
Compounds carried by water as soluble constituents may precipitate and
form scale as a result of pressure drop, temperature change, flow velocity
alterations, pH or alkalinity change or other water conditions.
Studies have shown that crystallization from solution of a material is
influenced by the following factors:
Supersaturation
A saturated solution is one that is in equilibrium
with its solute. Supersaturated solutions are solutions that contain higher
concentrations of dissolved solute than their equilibrium concentration.
When the concentrations of the scaling ions exceed their solubility product,
scaling begins. Solutions can become supersaturated by a change in
temperature. a change in pH, addition of solid seeding material, evaporation
or pressure change. Thus, if (Ca") x (CO,-') is greater than the Ks p for
CACO-, at a given temperature, calcium carbonate will precipitate
CONTROLLING PARAMETERS
Time
Temperature
pH
Pressure
Velocity
Particle Size
Other Environmental Factors
Nucleation
Nucleation, the initial formation of a precipitate can
occur for many reasons. Corrosion products, oxide films, surface
imperfection, slime masses and bacterial growths can form as nucleation
sites. The nucleation occurs rapidly at high degrees of supersaturation.
These nuclei serve as the starting point for additional crystal growth
leading to scale formation.
Contact Time
For scale to form after a solution has become
supersaturated and nucleation has occurred, there must be sufficient
contact time between the solution and the nucleating sites on the surface.
Generally, the longer the contact time, the more likely the scale formation
becomes.
Adherence
Corroding surfaces are more likely to promote scale
deposition than noncorroding surfaces. Studies using polished surfaces
indicate that microscopic roughness, whether natural or produced by
corrosion; makes subsequent scale deposits more adherent.
Crystal Growth
Although the solubility limit must be exceeded for
scale to form, the rate of scale formation is controlled by the presence
or absence of scale inhibitors and other factors. The rate of crystal growth
and the rate of inhibition of crystal growth can be studied by monitoring
the amount of inhibitor required to keep the calcium level constant during
periods when solubility limits are exceeded (i.e., during scale formation),
CONTROLLING PARAMETERS
Flow Velocity
Surface Temperatures
Bulk Temperatures
Water Composition
Molecular & Atomic Ordering
Diffusion
Other Factors
Exposure to Localized High Temperature
Because the solubility of most calcium and magnesium salts decreases with an increase in
temperature, these salts tend to form scale on heat transfer surfaces where
the metal skin temperature is greater than the bulk water temperature.
Changes in pH or Alkalinity
Changes in the pH or alkalinity of
the cooling water may also have a major effect on the solubility of scaling
ions. An increase in alkalinity decreases the solubility of calcium
carbonate and also affects the solubility of calcium and iron phosphates.
An increase in pH also decreases the solubility of most calcium salts, with
the exception of calcium sulfate.
Addition of Sulfuric Acid for pH Control
Using sulfuric acid for pH control can cause high sulfate levels and increases the probability
of calcium sulfate scaling. Hydrochloric acid should be used if calcium
sulfate is a potential problem.
Addition of Corrosion Inhibitors and/or Scale Inhibitors
High concentrations of some corrosion inhibitors and scale inhibitors may
cause deposits. Polyphosphate inhibitors, for example, may hydrolyze and
revert to orthophosphate, leading to deposition of calcium or iron phosphate.
The overfeed of some phosphonates may lead to the formation of calcium
phosphate, because phosphonates can revert to orthophosphate.
Uncommon Ion Effect
The solubility of most slightly soluble salts
can be increased by the addition of sodium chloride and nitrate ions.
This increase in solubility is known as the uncommon ion effect.
Common Types of Scale
The following is a discussion of the common types of scale. Although they
will be dealt with individually, deposits that form in cooling water systems
are rarely homogenous. Invariably, deposits consist of a mixture of
water-formed scale deposits that can act as a cement to incorporate silt
and other foulants into the overall deposit
Calcium Carbonate (CaCO3) Scale
Calcium carbonate forms a dense, extremely adherent deposit. It is by far
the most common scale problem in cooling systems. Most natural waters
contain bicarbonate alkalinity and are at a pH<8.2. At a pH>8,2, free
carbon dioxide ceases to exist. While calcium bicarbonate is soluble in
most natural waters, calcium bicarbonate will be converted to calcium
carbonate if the pH and/or the water temperature is raised. On heating, the
following reaction occurs:
The tendency to form calcium carbonate can be predicted qualitatively by
the Langelier Index, the Ryznar Index, or the Stiff and Davis Index, all
discussed below.
Calcium Sulfate (CaSO4) Scale
The most common form of calcium sulfate is gypsum (CaSO4 -2H20), which is
approximately 50 times more soluble than calcium carbonate at 100 F
(37.78 C). Above 100 F, the solubility of calcium sulfate decreases as the
water temperature increases.
Calcium sulfate should not present a scale problem in a cooling system using
proper blowdown procedures. The higher solubility of calcium sulfate is the
basis for scale control by pH adjustment with sulfuric acid.
Without exceeding the solubility limits of CACO,, sulfuric acid feed allows
the cooling tower to operate at higher cycles of concentration. This method
of scale control can lead to calcium sulfate deposition if excessive amounts
of sulfuric acid are used for pH control, or if the makeup water is high in
sulfates. The solubility product for calcium sulfate is 1.95 x 10-4.
To prevent calcium sulfate scale, the sum of the calcium concentration
(as CaCO3,) and the sulfate concentration (as CaCO3,) must be less than
1,500 and their product should be kept below 500,000. With chemical
treatment by a phosphonate, the sum of calcium (as CaCO3) and the sulfate
(as CaCO3) can be as high as 2,500, and their product should be less than
1,500,000.
Calcium Phosphate Scale
If polyphosphate is being used alone, or in combination with zinc or
zinc/chromate for corrosion protection, the polyphosphate ions may hydrolyze
and revert to orthophosphate ions. Orthophosphate levels can also be
contributed to by reversion of phosphonates or naturally from the makeup
water.
However, phosphate scales can occur if conditions are favorable for
tricalcium phosphate (Ca3(PO4)2) since the solubility product for calcium
phosphate is very small. Temperature, pH, calcium and orthophosphate
concentrations affect the formation of calcium phosphate. Calcium
orthophosphate scale is more insulating than calcium carbonate, and thus
causes rapid heat transfer loss.
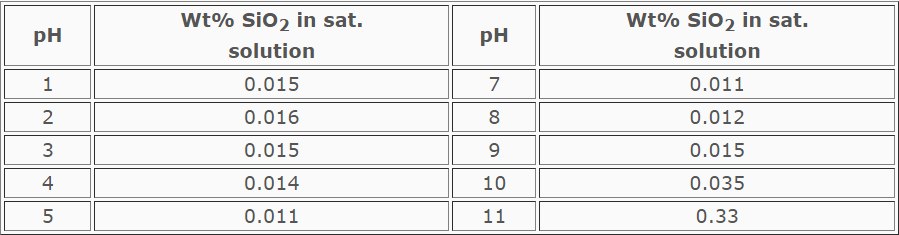
Silica Scale
The solubility of silica in water is high, so pure silica scale (SiO2)
is rare in cooling systems. The maximum amount that can be maintained in
solution in cooling systems is approximately 200 ppm. The higher the pH,
the more silica will stay in solution (see Table below). Silica may
coprecipitate with iron, manganese, aluminum and magnesium hydroxides.
TABLE
Solubility of SiO2 at Various pH Levels
Magnesium Silicate Scale
Soluble silica reacts readily with cations to form various complex silicates.
If magnesium is present in high enough concentrations. magnesium silicate
scaling will occur at pH of 8.5. Deposition of magnesium silicate can be
prevented by keeping
1. (Mg ppm as CaCO3) x (SiO2 ppm) <35,000 ppm @ pH<7.5
2. (Mg ppm as CaCO3) + (SiO2 ppm) <17,000 ppm @ pH>7.5
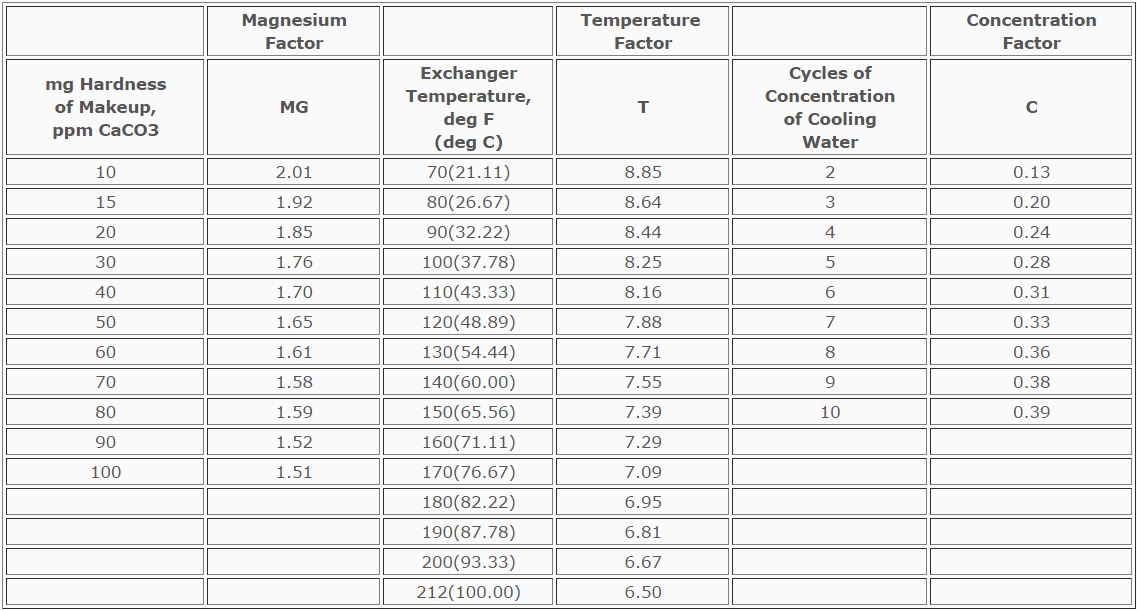
or more generally by keeping silica levels <200 ppm in the recirculating
water. Magnesium silicates form by a two-step process: First, magnesium
hydroxide precipitates, then reacts with dissolved and colloidal silica to
form scale. Magnesium silicate scale occurs when the pH level is higher
than 7.8. Magnesium silicate does not occur if the pH of the circulating
water is lower than the pH of saturation of magnesium hydroxide. This pH
of saturation can be determined using the Table below, which is also helpful
in predicting the deposition of magnesium silicate.
TABLE
Prediction of Magnesium Silicate Scale Deposition
pHs = MG + T - C
Example: MgH of makeup = 20 ppm, Cycles of concentration = 10 and pH of
circulating water = 8.7
Calculation of pHs at temperatures of 130, 150, 170, and 190 deg F:
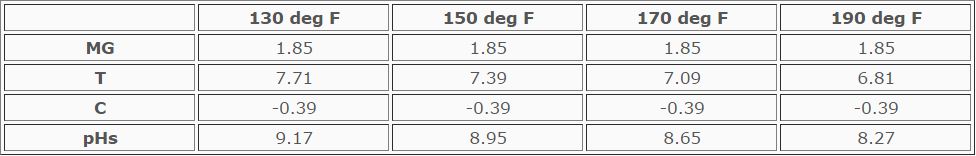
This water would deposit magnesium silicate in coolers whose temperature
exceeds 170 deg F.
Iron-Based Scales
Iron exists in two states, Fe+2 and Fe+3. The chemistry of iron compounds
is considerably more complex than alkaline earth carbonate and sulfate
scale-forming materials. When these two oxidation states (Fe+2 and Fe+3)
of iron join with the same anion, the result is usually the formation of
compounds with significant differences in solubilities. Iron deposits
typically are in the form of Fe2O3, FeO, FeS, iron silicate, etc. Often
manganese is also detected in these deposits.
Iron fouling occurs as a result of corrosion processes throughout the system.
Thus, it is very important to implement a good corrosion control program.
Iron fouling sometimes occurs in cooling waters as a result of carryover
from clarifiers, where iron salts may be used as coagulants or where raw
water (such as well water) may be high in iron. Iron levels of 2 ppm or
greater in the recirculating water can be controlled through the use of
iron dispersants or deposit control agents. Use of an iron dispersant is
strongly recommended to avoid major equipment failure problems. In cooling
waters, Fe2O3 (hematite) and FeO are the most common iron deposits.
Magnetite (Fe3O4) is rarely found in cooling systems. Magnetite needs high
temperatures and/or anaerobic conditions. Most magnetite found in cooling
systems arrives via airborne or waterborne solids. If iron levels exceed
4-5 mg/l, mechanical or other means to remove iron should be strongly
considered, such as oxidation, aeration, coagulation, etc.
Zinc Phosphate and Zinc Hydroxide
Zinc hydroxide scale is normally found when pH exceeds 7.6 or when over-feed
of a zinc product occurs. Zinc hydroxide is a grayish-white precipitate.
With increasing pH, precipitate amounts increase. As pH is lowered below
7.0, zinc hydroxide will resolubilize. Above 140 deg F (60 deg C) it has
an inverse temperature solubility.. The most common methods to prevent
scale in cooling water systems are:
using scale inhibitors.
adjusting pH.
softening makeup water.
Estimating the Scale-Forming Tendency of Water
Scale forming and/or corrosive tendencies of water can be predicted
qualitatively by using the Langelier Index, Ryznar Solubility Index, or
the Stiff and Davis Index.
The Langelier Index:
For any given temperature and water composition,
one can calculate the tendency of the water to form calcium carbonate scale
by using the Langelier Index.
LSI = pH - pHs
where
pHs = pCa + palk + C
where
pHs = saturated pH
pCa = calcium hardness factor (expressed as ppm CACO3,)
palk = M alkalinity factor (expressed as ppm CACO3,)
C = total solids (expressed as ppm at the temperature of the water)
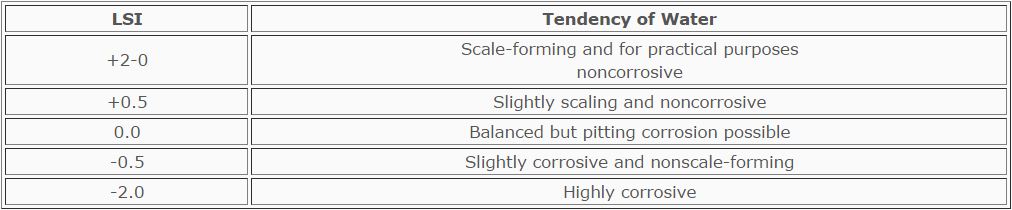
The Langelier Saturation Index (LSI) is defined as the difference between
the actual pH and the calculated saturated pH (pHs),
LSI = pH (actual) - pH (calculated)
LSI = pH - pHs
When the actual pH is equal to the saturated pH, the Langelier Saturation
Index is zero (LSI = 0 when pH = pHs). A saturation equilibrium exists.
There is no scale formation and corrosive attack is minimized.
When the actual pH is greater than the saturated pH, the Langelier
Saturation Index is positive (LSI when pH>pHs). Supersaturation of CACO3,
exists with respect to alkalinity and total solids at that temperature.
There is a tendency to form scale on heat transfer surfaces-
When the actual pH is less than the saturated pH, the Langelier Saturation
Index is negative (LSI = - when pH<pHs.). Any scale previously formed will
be dissolved. Corrosion of unprotected or bare metal will occur.
The Langelier Index does not tell whether scale will actually form, nor
how rapidly it will form.
The prediction of water characteristics by LSI appears in the Table below:
TABLE
Prediction of Water Characteristics by the Langelier Index
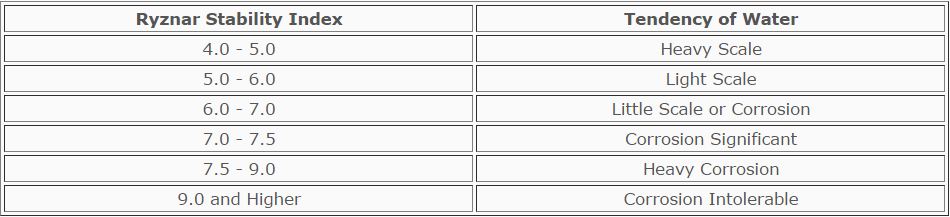
Ryznar Stability Index (RSI)
It is possible for a low-hardness water and a high-hardness water to have the same LSI value.
Using operational data and experience with scale and corrosion in a large
number of systems, Ryznar refined the Langelier Index to allow for a
distinction between two waters having the same Langelier Index. The
Ryznar equation is:
Ryznar Stability Index (RSI) = 2pHs - pH
The predictive nature of the Ryznar Index is shown in the Table below:
TABLE
Prediction of Water Characteristics by the Ryznar Index
The Stiff and Davis Index (SDI):
The Langelier Index has
been further modified by Stiff and Davis. Their index takes into account
the influence of high levels of dissolved solids on the solubility of
calcium carbonate. The SDI Index was developed for use in the oil field,
where highly saline waters or brines are produced. Where fresh water is
being used as makeup water for a cooling system, there is no need for the
refinements of the Stiff and Davis Index. However, as zero blowdown systems
become more common, as more and more reuse of water becomes necessary, and
as recovered wastewater is used in larger quantities as a makeup source, it
may become necessary to use the Stiff and Davis Index. The index is defined
as:
SDI = pH - pCa - pAlk - K
K = a constant based on the total ionic strength and temperature.
Methods of Scale Inhibition
Lime or Lime/Soda Softening
For cooling water applications, only partial lime or lime/soda softening is
economical. Often, sidestream lime softening is used as a supplement or
replacement for softening during external pretreatment prior to cooling
water applications. Occasionally, hot process lime or lime/soda softening
is used for silica removal in cooling water applications. In boiler
applications, however, hot or cold lime-soda softening is commonly used
for external treatment. This reaction is as follows:
2HCO3- + Ca(OH)2 --> CaCO3(s) + CO3-2 + 2H20
(M alkalinity)
Mg+2 + Ca(OH)2 --> Mg(OH)2(s) + Ca+2
Ca+2 + CO3-2 --> CaCO3(s)
Acid Treatment
The traditional method of calcium carbonate scale control is to reduce the
water's alkalinity sufficiently with sulfuric acid to create non-scaling
conditions.
Ca+2 + 2HCO3- + 2H++SO4-2 --> Ca+2+SO4-2 + 2CO2(g) + 2H20
The calcium bicarbonate is converted to volatile carbon dioxide and calcium
sulfate. Calcium sulfate is more soluble than calcium carbonate. The
cycles of concentration are limited by the solubility of calcium sulfate.
If the makeup water is high in sulfates, hydrochloric acid may be used to
reduce the alkalinity-, but chloride ions may cause problems because they
can penetrate oxide films, setting up local anodic cells and causing
corrosion.
Scale Inhibitors and Dispersants
The answer to the question of how scale inhibitors work is fraught with
complexities. One of the more logical explanations is that sub-
stoichiometric amounts of certain types of chemical additives can have
very marked effects on the growth rate of crystals deposited in a scaling
environment. These threshold inhibitors function by adsorbing onto the
growing crystals and distorting the lattice, which disrupts the crystal
growth process. The most commonly used threshold scale inhibitors are
inorganic polyphosphates, organophosphorous compounds, synthetic organic
dispersants and natural organic dispersants. Threshold treatment is the
term that describes the effective use of a scale inhibitor at concentrations
below the level required to sequester, or donate electrons to the metal ions
forming a water-soluble metal ion complex. The dispersant products are also
used to prevent scale formation by modifying the crystal structure of
the deposit-forming substance. This crystal distortion prevents deposition,
and the highly irregular stressed crystals tend to slough off as crystal
growth occurs.
Conventionally, scale inhibitors are classified as inorganic scale inhibitors
(such as sodium hexametaphosphate, sodium polyphosphate, sodium pyrophosphate)
or organic phosphorus compounds (such as the phosphate esters and
phosphonates). The inorganic phosphates used to treat scale contain
repeating oxygen/phosphorus bonds (-O-P-0-PO-P-). These bonds are highly
unstable in aqueous solutions, and as a result, they hydrolyze, or react
with water, and end up as ineffective orthophosphate. The hydrolysis of
such products is also referred to as reversion. Organic phosphorus
products are also subject to hydrolysis. The main factors that effect the
rate of reversion are pH and temperature. It is also influenced by
complexing cations, concentrations, holding time index, ionic environment
in the solution, and other factors. Environmental restrictions imposed upon
the industry have made organic treatments almost a necessity. The following
discussion about organic scale inhibitors and dispersants will be useful in
understanding the types of products.
Phosphate Esters
A typical phosphate ester functional group may be represented as follows:
O
||
R - C H 2 - O - P - O H
|
O H
Phosphate esters are less likely to hydrolyze than inorganic polyphosphates
and more likely to hydrolyze than phosphonates. They exhibit varying degrees
of threshold scale inhibitor properties. In fact, some phosphate esters are
ineffective as scale inhibitors.
Phosphonates
A typical phosphonate has the following structure:
O
||
R - C H 2 - P - O -
|
O -
This structure with C-P-0 bonding is more stable to hydrolysis than the
phosphate ester.
A variety of amino methylene phosphonic acids and their salts are available
commercially as scale inhibitors. They are effective inhibitors for both
calcium sulfate and calcium carbonate scale at very low threshold
concentrations. Non-nitrogen-containing phosphonic acids are widely used
because they are more compatible with chlorine than the aminomethylene
phosphonic acids. Phosphonates are believed to inhibit scale formation by
being adsorbed on active crystal growth sites, where they decrease the
crystal growth rate and decrease the nucleation rate. A number of blends
or combination products that incorporate low molecular weight dispersant
polymers with phosphonates are available. Phosphonates containing nitrogen
are good for iron control because the close proximity of the nitrogens allows
for formation of strong five-membered ring metal complexes with iron. The
structure of a typical phosphonate is as follows:
HEDPA
OH OH OH
| | |
HO - P - C - P - OH
|| | ||
O CH3 O
AMP
/C H 2 P O 3 H
N - C H 2 P O 3 H
C H 2 P O 3 H
Synthetic Polymeric Dispersants
A large number of synthetic polymers are used as scale inhibitors and as
dispersants. Most of the synthetic polymers have a molecular weight
below 50,000 and are polymers of acrylic acid, acrylamide, methacrylic acid,
maleic acid, maleic anhydride, styrene maleic blends and maleics
(methylvinyl ether), etc. The effectiveness of the polymer decreases as the
chain length increases. A length of about 10-15 repeating units is best.
At this molecular weight, adsorption is maximized without causing bridging,
and dispersion is most efficient. Synthetic polymers such as polyacrylates
are believed to inhibit scale deposition by adsorption on growing crystals.
This is followed by both crystal structure modification and by a charge
repulsion mechanism of negative charges, which leads to destabilization of
the crystallites. Polymers are also used for iron control.
Natural Organic Polymers - Lignosulfonates
Lignosulfonate derivatives come from pulsing operations where wood is
sulfonated at high temperatures to remove the lignin binder from the
cellulose by forming a water-soluble polymer. This polymer is a highly
complex natural derivative and its structure is not well defined.
Lignosulfonates are classified as anionic polyelectrolytes. Those used
as dispersants usually have a molecular weight between 1,000 and 10,000.
Lignosulfonates are usually poor scale inhibitors. However, these organic
polymers are reported to be good dispersants for calcium phosphate,
suspended matter and iron oxides. Lignosulfonates function as dispersants
by limiting attractive forces between particles, thereby reducing
sedimentation.
Inorganic Polyphosphates
Polyphosphates are commonly used in once-through and municipal applications
to minimize scaling and corrosion at dosages of 1 to 5 mg/l of polyphosphate
as PO4. Thus, polyphosphates function as threshold treatments because they
are effective at dosages much less than those stoichiometrically required to
react with calcium, magnesium or iron. The repeating (P-0-P) structure is
characteristic of inorganic polyphosphates.
Although the advantages of low cost and threshold activity are attractive
for the use of polyphosphates, they are ineffective as scale inhibitors at
high levels of calcium. High iron and manganese levels can restrict the
effectiveness of polyphosphates by forming stoichiometric iron-polyphosphate
complexes that hinder the performance of polyphosphates as inhibitors for
calcium and magnesium deposits. In addition, polyphosphates act as nutrients
for bacteria. As a result, higher biocide dosages may be required if
polyphosphates are used for scale or corrosion control.
Surfactants
Surfactants are used in cooling systems either to emulsify or disperse
hydrocarbons, or to penetrate biomasses. Their primary purpose is to
prevent hydrocarbon and microbial deposition on heat transfer surfaces.
Surfactants also aid in de-oiling surfaces and keeping suspended solids,
including biomass, dispersed. Surfactant types are generally classified
as anionic, cationic, nonionic and amphoteric.
Surfactants work in one of two ways. If they are nonionic or amphoteric,
they reduce surface tension or interfacial tension at liquid-solid,
liquid-air, liquid-liquid and solid-air interfaces. This changes the
wetting characteristics of solids, making them either water-wet or oil-wet.
If the surfactants are anionic or cationic, they work by charge enhancing
the solids, either oil or biomass, and forcing those solids to repel one
another. This keeps the solids dispersed in the system water for later
removal. Reduction of interfacial tension is also achieved through the
use of anionic and cationic surfactants.
Problems Caused by Scale or Fouling Deposits in
Cooling Systems
Reduced or uneven heat transfer
Unexpected equipment shutdown - loss of production
Shortened equipment life
Increased pumping costs
Equipment corrosion
Product loss due to ineffective cooling
Increased product cost due to above factors
Profit Loss


