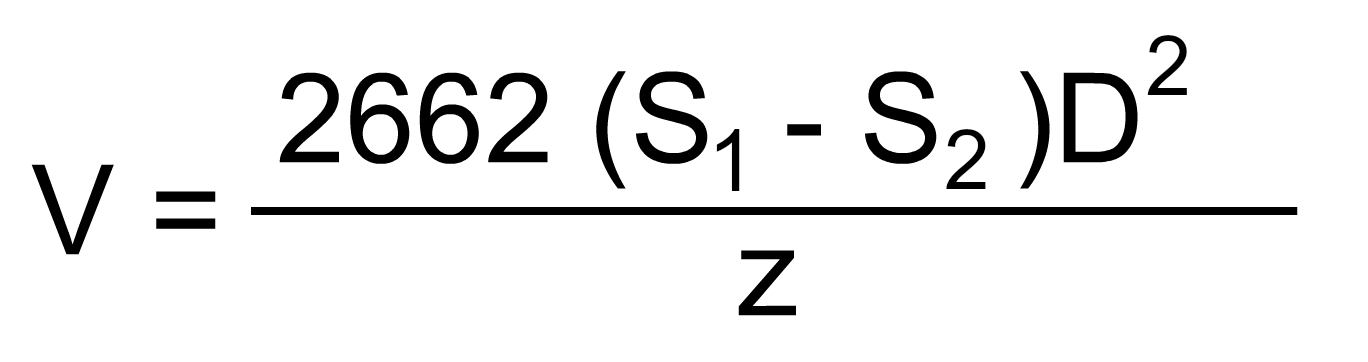GC³ Technical Manual: Clarification
GC³ Technical Manual: Clarification
Introduction
Water clarification is the process of removing suspended solids from water. In this chapter, factors governing the destabilization, coagulation and removal of suspended solids from water are reviewed. Important concepts of this section include:
1. Stable solids suspensions in water-The mechanisms involved in keeping solids suspended in water
2. Chemical treatments-How organic polymers and inorganic coagulants work to counteract solids stabilization mechanisms and enhance removal of solids from water
3. The function of clarification unit operations-How these units work and how chemical treatment enhances their performance
After reading this chapter, you should have the basic information required to begin on-site investigations of water clarification processes. You should be able to define and discuss coagulation, flocculation, chemical treatment and clarification processes as applied to the physical treatment of surface waters.

Subsidence
While a degree of clarification can be accomplished by subsidence (settling), most industrial processes require better quality water than can be obtained from subsidence only. Most of the suspended matter in water would settle, given enough time, but in most cases the amount of time required would not be practical. The time required for settling is dependent on many factors, including:
1. Weight of the particle
2. Shape of the particle
3. Size of the particle
4. Viscosity and/or frictional resistance of the water, which is a function of temperature
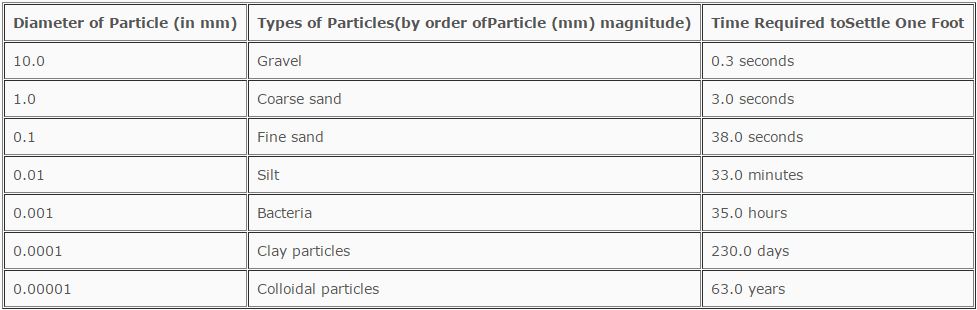
The settling rates of various size particles at 50 ºF (10 ºC) is illustrated in Table 6.1.
TABLE 6.1
Settling Rates by Particle Size
Stokes' Law
Settling velocities may be calculated from Stokes' Law.
Where V = Velocity of fall (ft/sec)
D = Diameter of particle (in)
S1= Density of particle (lb/ft3)
S2= Density of fluid (lb/ft3)
z = Viscosity (centipoises)
In this equation it is assumed that the particles are spherical, failing under viscous resistance, and that they have no electrostatic charges. This is, of course, never true under actual conditions. Most suspended solids smaller than 0.1 mm found in surface waters carry negative electrostatic charges. This charge causes the particles to repel each other, increasing their stability and thus increasing their tendency to remain suspended. Chemicals are often added to water to neutralize particle charge and enhance particle settling. Chemicals used to promote suspended particle subsidence in the clarification process are commonly called coagulants; the particle charge neutralization process is called coagulation.
Coagulation
Coagulation, the first step in complete clarification, is the neutralization of the electrostatic charges on colloidal particles. Because most of the smaller suspended solids in surface waters carry a negative electrostatic charge, the natural repulsion of these similar charges causes the particles to remain dispersed almost indefinitely. To allow these small suspended solids to agglomerate, the negative electrostatic charges must be neutralized. This is accomplished by using inorganic coagulants (water soluble inorganic compounds), organic cationic polymers or polyelectrolytes.
Inorganic Coagulants
The most common inorganic coagulants are:
-
Alum-aluminum sulfate-Al2(SO4)3
-
Ferric sulfate-Fe2(SO4)3
-
Ferric chloride-FeCl3
-
Sodium aluminate-Na2AI204
Inorganic salts of metals work by two mechanisms in water clarification. The positive charge of the metals serves to neutralize the negative charges on the turbidity particles. The metal salts also form insoluble metal hydroxides which are gelatinous and tend to agglomerate the neutralized particles. The most common coagulation reactions are as follows:
Coagulation Reactions
Al2(SO4)3 + 3Ca(HCO3)2 = 2Al(OH)3 + 3CaSO4 + 6CO2
Al2(SO4)3 + 3Na2CO3 + 3H2O = 2AI(OH)3 + 3Na2SO4 + 3CO2
Al2(SO4)3 + 6NaOH = 2AI(OH)3 + 3Na2SO4
Al2(SO4)3 (NH4)2SO4 + 3Ca(HC03) = 2AI(OH)3 + (NH4)2SO4 + 3CaSO4 + 6CO2
Al2(SO4)3 K2SO4 + 3Ca(HCO3)2 = 2AI(OH)3 + K2SO4 + 3CaSO4 + 6CO2
Na2AI204 + Ca(HCO3)2 + H20 = 2AI(OH)3 + CaCO3 + Na2CO2
Fe(SO4)3 + 3Ca(OH)2 = 2Fe(OH)3 + 3CaSO4
4Fe(OH)2 + O2 + 2H2O = 4Fe(OH)3
Fe2(SO4)3 + 3Ca(HCO3) = 2Fe(OH)3 + 3CaSO4 + 6CO2
Coagulant Equivalents
The effectiveness of inorganic coagulants is dependent upon water chemistry (pH and alkalinity), and their addition usually alters that chemistry. Table 6.2 illustrates the effect of the addition of 1 ppm of the various inorganic coagulants.
TABLE 6.2
Coagulant, Acid and Sulfate-1 ppm Equivalents
Alkalinity Relationships
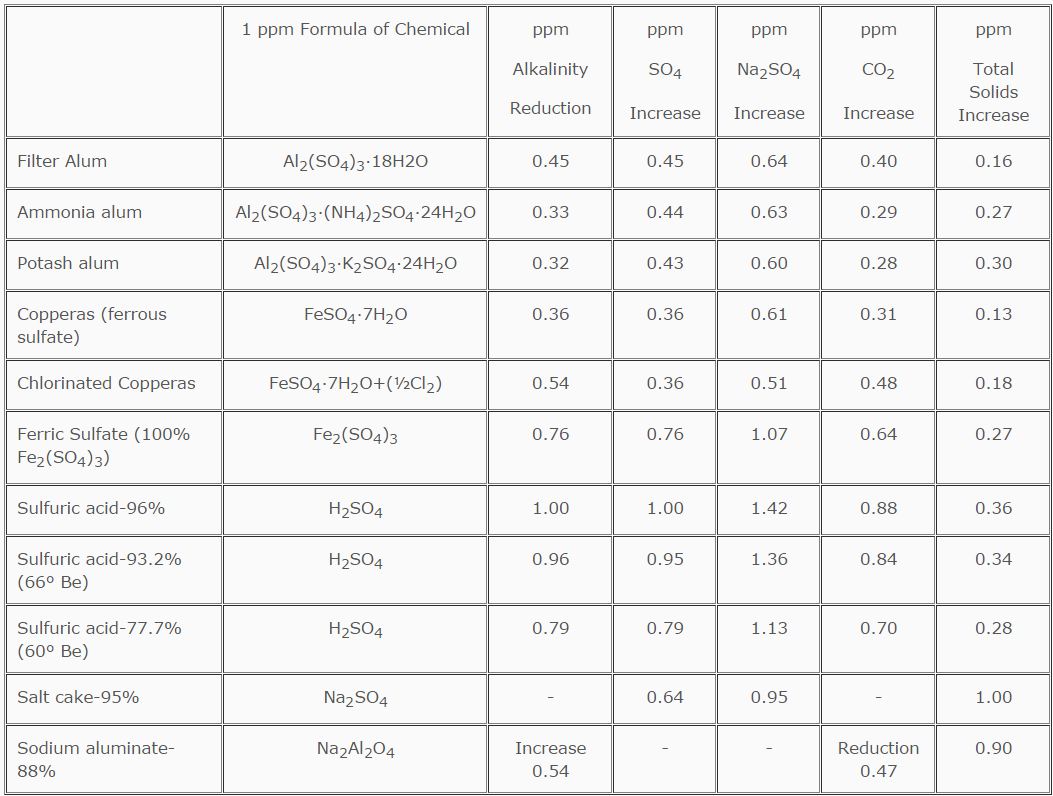
Aluminum salts are most effective as coagulants in a 5.5-8.0 pH range. Because they react with the alkalinity in the water, it may be necessary to add additional alkalinity in the form of lime or soda ash. Iron salts, on the other hand, are most effective as coagulants at higher pH ranges (8-10). Iron salts also depress alkalinity and pH levels; therefore, additional alkalinity must be added. Sodium aluminate increases the alkalinity of water, so care must be taken not to exceed pH and alkalinity guidelines. As is evident from the reactions discussed above, a working knowledge of the alkalinity relationships of water is mandatory.
It is important to note, at this point, that the use of metal salts for coagulation may increase the quantity of dissolved solids. One must consider the downstream impact of these dissolved solids. In addition, the impact of carryover of suspended Al+++ and Fe+++ compounds and their related effect on downstream processes must be considered.
For example, if the alum demand of water is 50 ppm, the sulfate increase in the effluent water would be 50 ppm x .45, or 22.5 ppm. If an equivalent amount of lime had to be added, the dissolved solids content of the water would be further increased by
It must also be pointed out that using inorganic coagulants produces a voluminous, low-solids sludge that dewaters and dries very slowly.
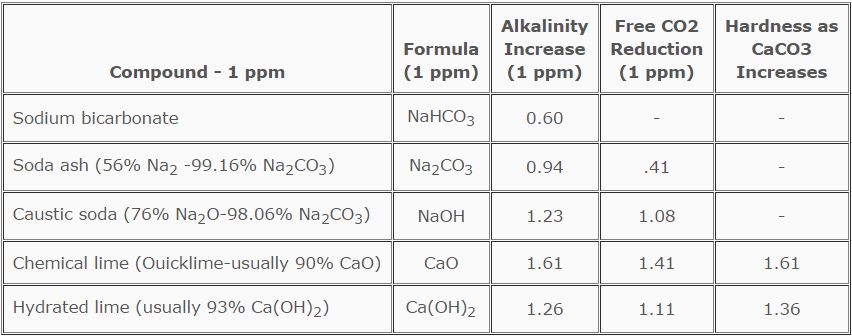
TABLE 6.3
Alkali and Lime 1 ppm Equivalents
Organic Coagulants - Polymers
While these coagulants serve the same function as the inorganic metal salts, the process is simpler because the charge neutralization reaction is the only concern. Polymer addition has no effect on pH or alkalinity, so no supplemental chemical feed is required to control either.
Polymers can be envisioned as long chains with molecular weights of 1000 or less to 5,000,000 or more. Along the chain are numerous charged sites. In primary coagulants, these sites are positively charged. The sites are available for adsorption onto the negatively charged particles in the water. To accomplish optimum polymer dispersion and polymer/particle contact, initial mixing intensity is critical. The mixing must be rapid and thorough. Polymers used for charge neutralization cannot be over-diluted or over-mixed. The farther upstream in the system these polymers can be added, the better their performance.
Because most polymers are viscous, they must be properly diluted before they are added to the influent water. Special mixers such as static mixers, mixing tees and specially designed chemical dilution and feed systems are all aids in polymer dilution.
Flocculation - The Second Step of the Coagulation Process
Once the negative charges of the suspended solids are neutralized, flocculation begins. Charge reduction increases the occurrence of particle-particle collisions, promoting particle agglomeration. Portions of the polymer molecules not absorbed protrude for some distance into the solution and are available to react with adjacent particles, promoting flocculation. Bridging of neutralized particles can also occur when two or more turbidity particles with a polymer chain attached come together. It is important to remember that during this step, when particles are colliding and forming larger aggregates, mixing energy should be great enough to cause particle collisions but not so great as to break up these aggregates as they are formed.
In some cases flocculation aids are employed to promote faster and better flocculation. These flocculation aids are normally high molecular weight anionic polymers. Flocculation aids are normally necessary for primary coagulants and water sources that form very small particles upon coagulation. A good example of this is water that is low in turbidity but high in color (colloidal suspension).
Color Removal
By far the most difficult impurity to remove from most surface waters is color (from dissolved or colloidal suspensions of decayed vegetation) and other colloidal suspensions.
Color in surface water normally is a result of its contact with decayed vegetation and is composed of tannins and lignins, the components that hold together the cellulose cells in vegetation. In addition to their undesirable appearance in drinking water, these organics can cause serious problems in downstream water purification processes. For example:
-
Expensive demineralizer resins can be irreversibly fouled by these materiais.
-
Some of these organics have chelated trace metals, such as iron and manganese within their structure, which can cause serious deposition problems in a cooling system.
There are many ways of optimizing color removal in a clarifier:
-
Prechlorination (before the clarifier) significantly improves the removal of organics as well as reducing the coagulant demand.
-
The proper selection of polymers for coagulation has a significant impact on organic removal.
-
Color removal is affected by pH. Generally, organics are less soluble at low pH.
Should organic removal prove to be a problem, a relatively simple test procedure is available to determine removal efficiency. (Refer to Bench Testing Procedures Handbook.)
Clarification Process (Mechanical)
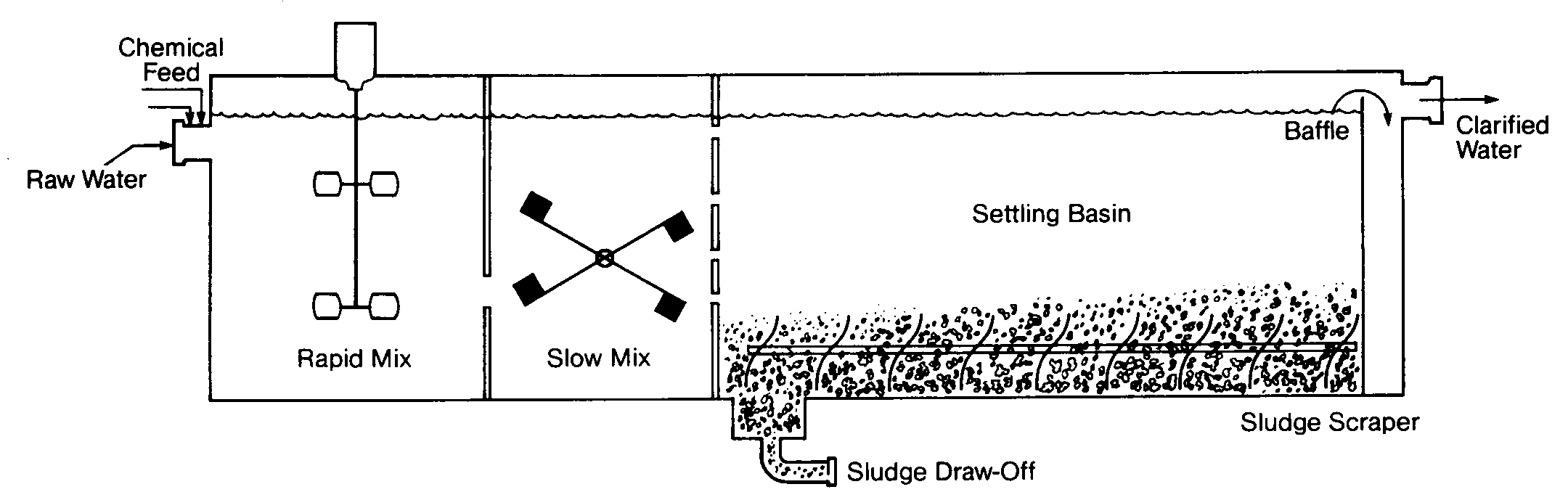
Conventional Clarification
The simplest form of clarification uses a large tank or horizontal basin for sedimentation of flocculated solids, as shown in Figures 6.1 and 6.2. The basin may contain separate chambers for rapid mix, slow mix and settling. The first two steps are important for good clarification. An initial period of turbulent mixing is necessary for contact between the coagulant and the suspended matter, followed by a period of gentle stirring to increase collisions between particles and increase floc size. Typical retention times are 3-5 minutes for rapid mix, 15 to 30 minutes for flocculation. and 4-6 hours for settling.
The coagulant is added to the waste water in the rapid mix chamber or just upstream, and the water passes through the mix chambers into the settling basin. As the water passes along the length of the basin (or out to the circumference in the case of a circular clarifier), the flocculated particles settle to the bottom and are scraped into a sludge collection basin for removal and disposal. Clear water flows over a weir and is usually held in a tank called a clearwell.
FIGURE 6.1
Large Tank Clarifier
FIGURE 6.2
Horizontal Basin Clarifier
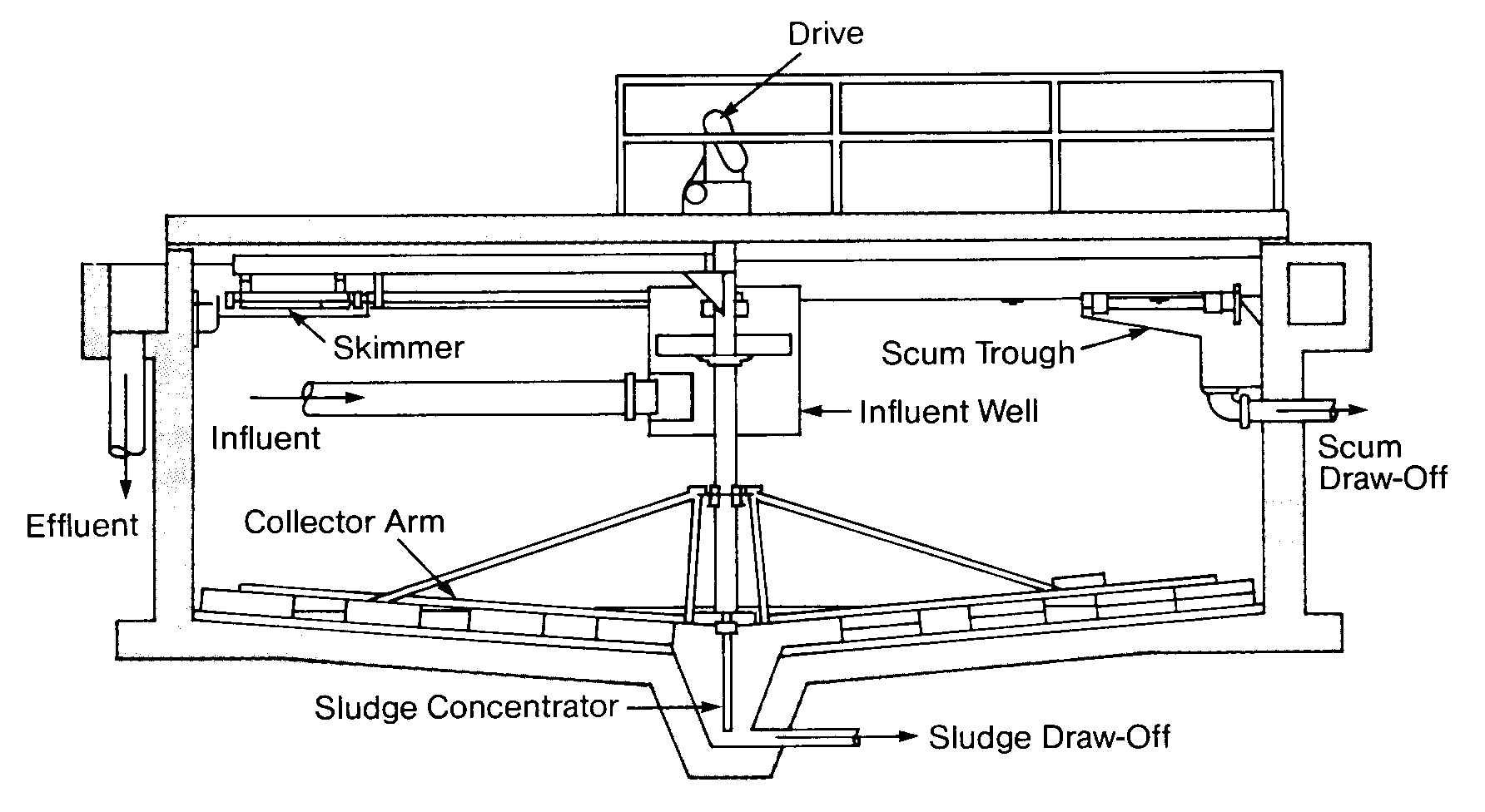
Upflow Clarification
It is often advantageous to employ a zone of high solids contact to achieve a better quality effluent. This is accomplished in an upflow clarifier, so called because the water flows upward through the clarifier as the solids settle to the bottom. Most upflow clarifiers are either solids-contact or sludge-blanket type clarifiers, which differ somewhat in theory of operation. Cross-sections of these two types of units are shown in Figures 6.3 and 6.4. Both units have an inverted cone within the clarifier. Inside the cone is a zone of rapid mixing and a zone of high solids concentration. The coagulant is added either in the rapid mix zone or somewhere upstream of the clarifier.
FIGURE 6.3
Solids-Contact Clarifier
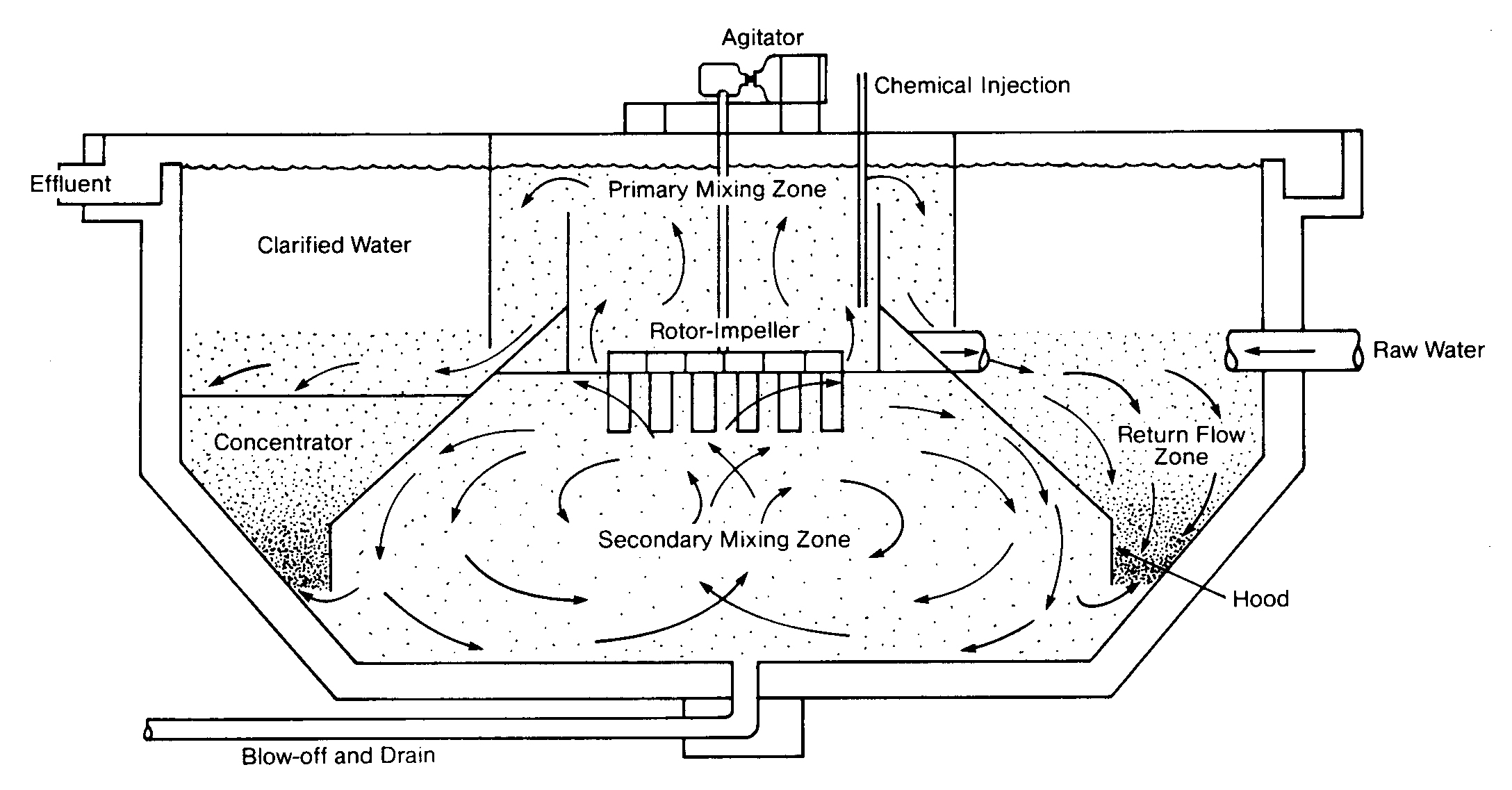
FIGURE 6.4
Sludge-Blanket Clarifier
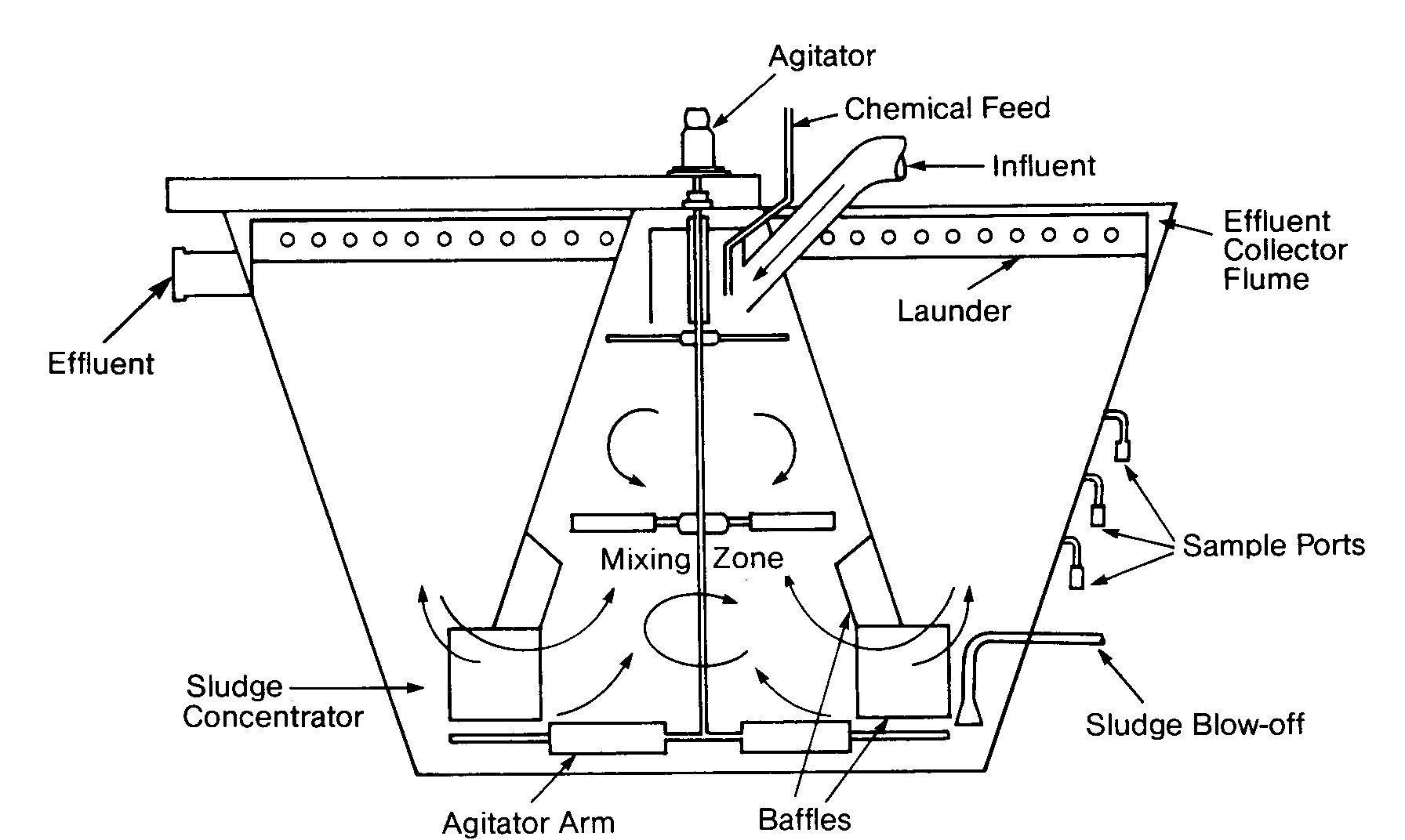
The Solids-Contact Clarifier
In the solids-contact clarifier, raw water is drawn into the primary mixing zone, where initial coagulation and flocculation take place. The secondary mixing zone is used to produce a large number of particle collisions so that smaller particles are entrained in the larger floc. Water passes out of the inverted cone into the settling zone, where solids settle to the bottom and clarified water flows over the weir. Solids are drawn back into the primary mixing zone, causing recirculation of the large floc. The concentration of solids in the mixing zones is controlled by occasional or continuous blowdown of sludge.
The Sludge-Blanket Clarifier
The sludge-blanket clarifier goes one step further, by passing the water up from the bottom of the clarifier through a blanket of suspended solids that acts as a filter. The inverted cone within the clarifier produces an increasing cross-sectional area from the bottom of the clarifier to the top. Thus, the upward velocity of the water decreases as it approaches the top. At some point, the upward velocity of the water exactly balances the downward velocity of a solid particle and the particle is suspended, with heavier particles suspended closer to the bottom. As the water containing flocculated solids passes up through this blanket, the particles are absorbed onto the larger floc, which increases the floc size and drops it down to a lower level. It eventually falls to the bottom of the clarifier to be recirculated or drawn off.
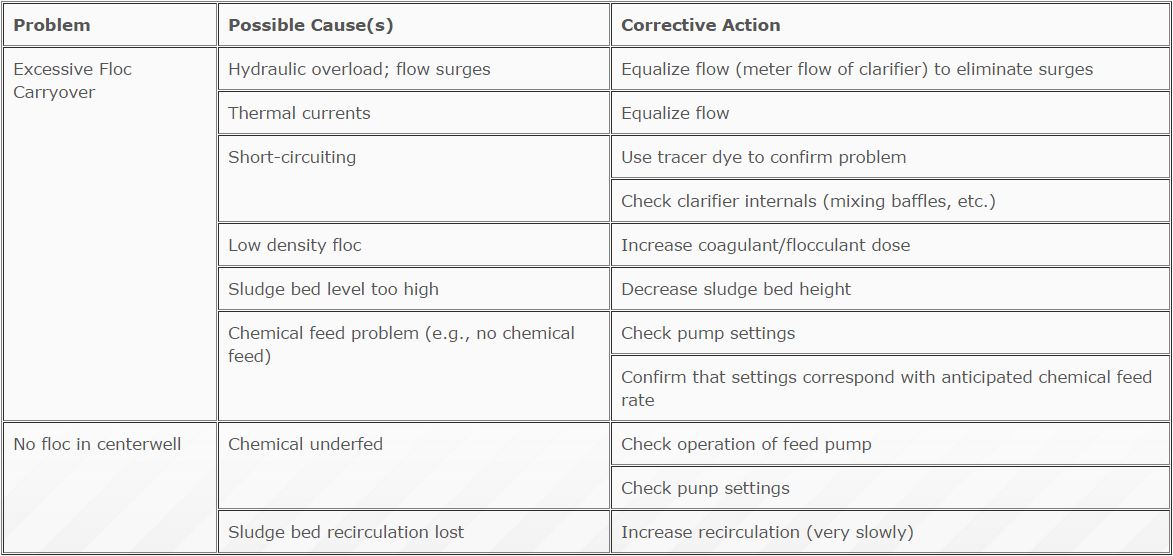
Troubleshooting Tips
Problems normally show up as floc or turbidity carryover from the clarifier. This problem can be caused by many factors, some of which are listed in Table 6.4.
Application Pointers
1. Always read the clarifier equipment manual for information about how the unit should be operated.
2. Remember that primary coagulants thrive on mixing.
3. Try different feed points.
4. Don't feed polymers too close to chlorine or other oxidants.
5. Use dilution water with polymers.
6. Optimize all variables (sludge bed depth, turbine speed, etc.).
7. Split up feeding of polymers.
8. Remember the sludge bed level will drop when polymer is first added. Watch the blowdown closely.
9. Learn to watch the centerwell for troubleshooting and to observe quickly the effect of any chemical dosage changes.
TABLE 6.4
Floc Problems and Solutions