GC³ Technical Manual: Boiler Water
Boiling Water Systems
Introduction
This chapter is designed to give you a general overview of steam boilers and associated equipment. When you complete this chapter, you should be familiar with the following:
1. Utilization of steam
2. Steam plant operation
3. Equipment considerations
4. How to chemically treat a boiler system
5. Problems encountered in operating boilers
6. General troubleshooting of boiler water systems

Role of Boilers in Plant Operation (Steam Generation)
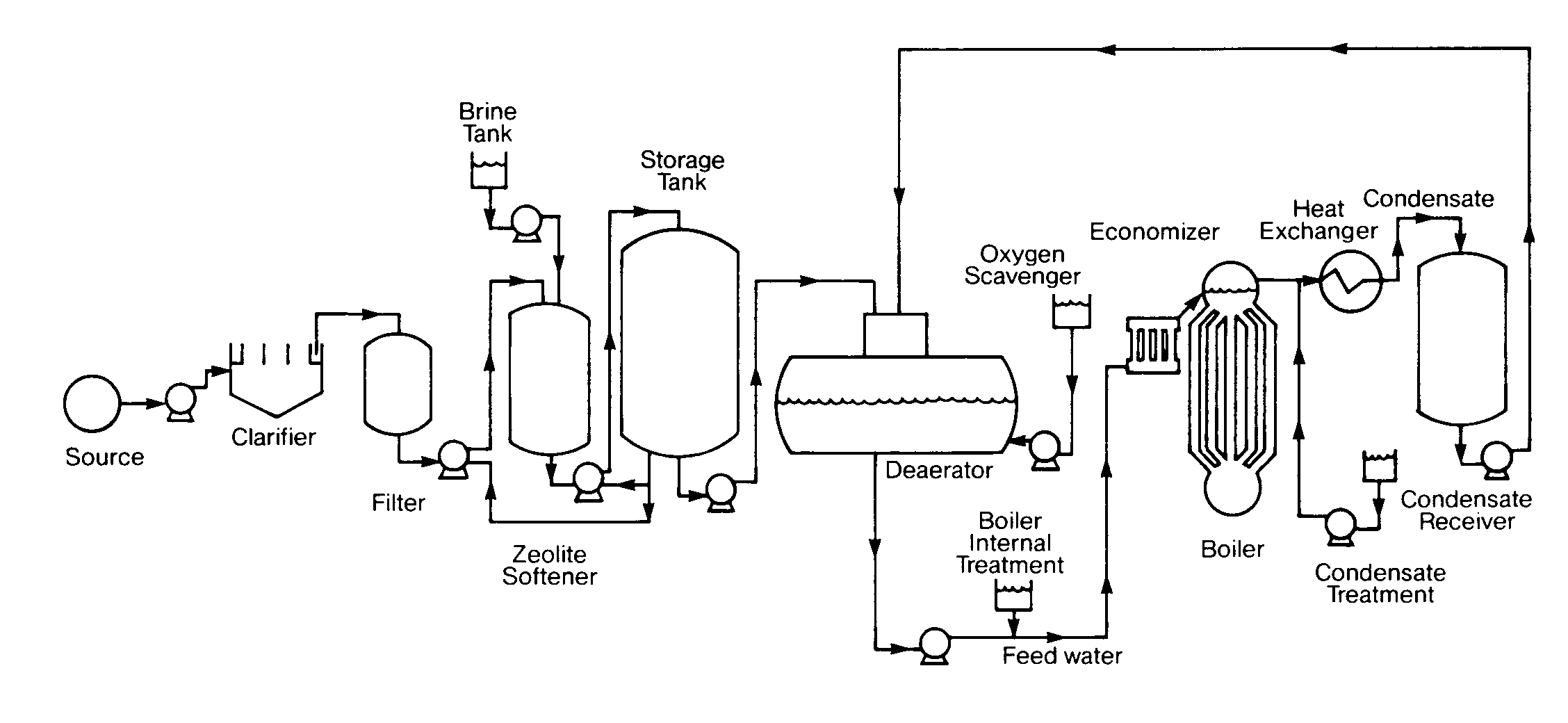
A boiler is a closed vessel in which water under pressure is transformed into steam by the application of heat. In the boiler furnace, the chemical energy in the fuel is converted into heat, and it is the function of the boiler to transfer this heat to the contained water in the most efficient manner. The boiler should also be designed to generate high quality steam for plant use. A flow diagram for a typical boiler plant is presented in Figure 12.l.
A boiler must be designed to absorb the maximum amount of heat released in the process of combustion. This heat is transferred to the boiler water through radiation, conduction and convection. The relative percentage of each is dependent upon the type of boiler, the designed heat transfer surface and the fuels.
FIGURE 12.1
Boiler Plant Flow Diagram
Types
Two principal types of boilers are used for industrial applications:
1. Fire tube boilers- Products of combustion pass through the tubes, which are surrounded by water.
2. Water tube boilers- Products of combustion pass around the tubes containing water. The tubes are interconnected to common channels or headers and eventually to a steam outlet for distribution to the plant system.
Utilization
The boiler house or steam generation facility within any given plant is frequently referred to as the heart. In the event this system shuts down for unexpected reasons or for plant turnaround, most processes within the plant will not be operable. For this reason, very conservative treatment measures are used in the boiler. Operating personnel can be reluctant to change treatment programs if the one currently in use is deemed successful. On the other hand, if a treatment program is linked to a boiler failure, change usually comes quickly.
Steam Utilization
Steam is generated for the following plant uses:
1. Turbine drive for electric generating equipment, blowers and pumps
2. Process for direct contact with products, direct contact sterilization and noncontact for processing temperatures
3. Heating and air conditioning for comfort and equipment
The efficiency achievable with steam generation relies heavily on the system's ability to return condensed steam to the operating cycle. Many of the systems described above return a significant portion of the condensed steam to the generation cycle.
The Role of Water Treatment in Steam Generation
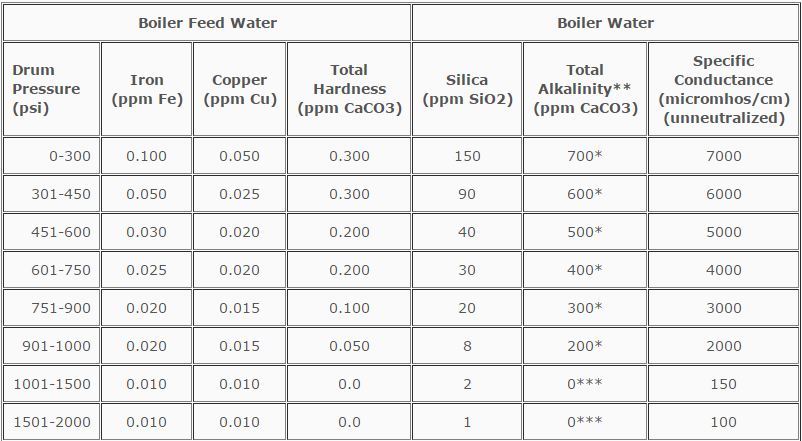
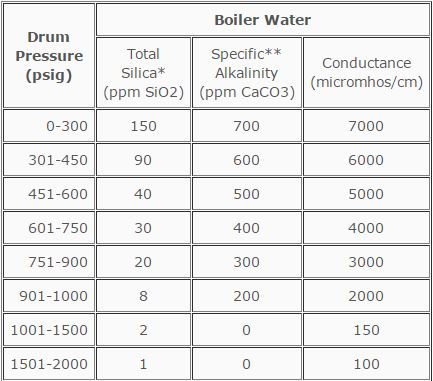
Based on an operating history that exceeds 50 years, the American Society of Mechanical Engineers (ASME) has provided guidelines for water quality in modern industrial boilers. These criteria were established to assure reliable and safe operation of boilers.
Table 12.1 truly reflects safe and reliable operation parameters. This is achieved through proper external treatment and proper internal treatment.
External Treatment
External treatment, as the term is applied to water prepared for use as boiler feed water, usually refers to the chemical and mechanical treatment of the water source. The goal is to improve the quality of this source prior to its use as boiler feed water, external to the operating boiler itself. Such external treatment normally includes:
1. Clarification
2. Filtration
3. Softening
4. Dealkalization
5. Demineralization
6. Deaeration
7. Heating
Any or all of these approaches can be used in feed water or boiler water preparation.
TABLE 12.1
ASME Guidelines for Water Quality in Modern Industrial Water Tube Boilers
for Reliable Continuous Operation
Internal Treatment
Even after the best and most appropriate external treatment of the water source, boiler feed water (including return condensate) still contains impurities that could adversely affect boiler operation. Internal boiler water treatment is then applied to minimize the potential problems and to avoid any catastrophic failure, regardless of external treatment malfunction.
Steam Plant Operation
For each plant operation, there is an optimum method of treatment. Many factors are involved in proper selection of feed water preparation and internal treatment. Principally, these are the requirements of the plant for safe and reliable operation at an economical treating cost.
Feed Water Preparation
This section deals with the preparation of boiler feed water. The basic assumption with regard to the quality of feed water is that calcium and magnesium hardness, migratory iron, migratory copper, colloidal silica and other contaminants have been reduced to a minimum, consistent with boiler design and operation parameters.
Once feed water quality has been optimized with regard to soluble and particulate contaminants. the next problem is corrosive gases. Dissolved oxygen and dissolved carbon dioxide are among the principal causes of corrosion in the boiler and pre-boiler systems. The deposition of these metallic oxides in the boiler is frequently more troublesome than the actual damage caused by the corrosion. Deposition is not only harmful in itself, but it offers an opening for further corrosion mechanisms as well.
Contaminant products in the feed water cycle up and concentrate in the boiler. As a result, deposition takes place on internal surfaces, particularly in high heat transfer areas, where it can be least tolerated. Metallic deposits act as insulators, which can cause local overheating and failure. Deposits can also restrict boiler water circulation. Reduced circulation can contribute to overheating, film boiling and accelerated deposition.
The best way to start to control pre-boiler corrosion and ultimate deposition in the boiler is to eliminate the contaminants from the feed water. Consequently, this section deals principally with the removal of oxygen, the
impact of trace amounts of contaminants remaining in the feed water, and heat exchange impact. Deposition and boiler corrosion are covered later in this chapter.
Feed water is defined as follows:
Feed water (FW) = Makeup water (MW) + Return condensate (RC)
The above equation is a mass balance (pounds or kilograms).
Deaeration (Mechanical and Chemical)
Mechanical and chemical deaeration is an integral part of modern boiler water protection and control. Deaeration, coupled with other aspects of external treatment, provides the best and highest quality feed water for boiler use.
Simply speaking, the purposes of deaeration are:
To remove oxygen, carbon dioxide and other noncondensable gases from feed water
To heat the incoming makeup water and return condensate to an optimum temperature for:
Minimizing solubility of the undesirable gases
Providing the highest temperature water for injection to the boiler
With this overview, a review of the potential problems, equipment and solutions in order.
Origin of the Problem
The most common source of corrosion in boiler systems is dissolved gas: oxygen, carbon dioxide and ammonia. Of these, oxygen is the most aggressive. The importance of eliminating oxygen as a source of pitting and iron deposition cannot be over-emphasized. Even small concentrations of this gas can cause serious corrosion problems.
Makeup water introduces appreciable amounts of oxygen into the system. Oxygen can also enter the feed water system from the condensate return system. Possible return line sources are direct air-leakage on the suction side of pumps, systems under vacuum, the breathing action of closed condensate receiving tanks, open condensate receiving tanks and leakage of nondeaerated water used for condensate pump seal and/or quench water. With all of these sources, good housekeeping is an essential part of the preventive program.
One of the most serious aspects of oxygen corrosion is that it occurs as pitting. This type of corrosion can produce failures even though only a relatively small amount of metal has been lost and the overall corrosion rate is relatively low. The degree of oxygen attack depends on the concentration of dissolved oxygen, the pH and the temperature of the water.
The influence of temperature on the corrosivity of dissolved oxygen is particularly important in closed heaters and economizers where the water temperature increases rapidly. Elevated temperature in itself does not cause corrosion. Small concentrations of oxygen at elevated temperatures do cause severe problems. This temperature rise provides the driving force that accelerates the reaction so that even small quantities of dissolved oxygen can cause serious corrosion.
The Corrosion Process
Localized attack on metal can result in a forced shutdown. The prevention of a forced shutdown is the true aim of corrosion control.
Because boiler systems are constructed primarily of carbon steel and the heat transfer medium is water, the potential for corrosion is high. Iron is carried into the boiler in various forms of chemical composition and physical state. Most of the iron found in the boiler enters as iron oxide or hydroxide. Any soluble iron in the feed water is converted to the insoluble hydroxide when exposed to the high alkalinity and temperature in the boiler.
These iron compounds are divided roughly into two types, red iron oxide (Fe2O3) and black magnetic oxide (Fe3O4). The red oxide (hematite) is formed under oxidizing conditions that exist, for example, in the condensate system or in a boiler that is out of service. The black oxides (magnetite) are formed under reducing conditions that typically exist in an operating boiler.
Deaerators
Mechanical deaeration is the first step in eliminating oxygen and other corrosive gases from the feed water. Free carbon dioxide is also removed by deaeration, while combined carbon dioxide is released with the steam in the boiler and subsequently dissolves in the condensate. This can cause additional corrosion problems.
Because dissolved oxygen is a constant threat to boiler tube integrity, our discussion on the deaerator will be aimed at reducing the oxygen content of the feed water. The two major types of deaerators are the tray type and the spray type. In both cases, the major portion of gas removal is accomplished by spraying cold makeup water into a steam environment.
Tray-Type Deaerating Heaters
Tray-type deaerating heaters release dissolved gases in the incoming water by reducing it to a fine spray as it cascades over several rows of trays. The steam that makes intimate contact with the water droplets then scrubs the dissolved gases by its counter-current flow. The steam heats the water to within 3-5 º F of the steam saturation temperature and it should remove all but the very last traces of oxygen. The deaerated water then falls to the storage space below, where a steam blanket protects it from recontamination.
Nozzles and trays should be inspected regularly to insure that they are free of deposits and are in their proper position (Figures 12.2 and 12.3).
FIGURE 12.2
Tray-Type Deaerating Heater (Cochrane Corp.)
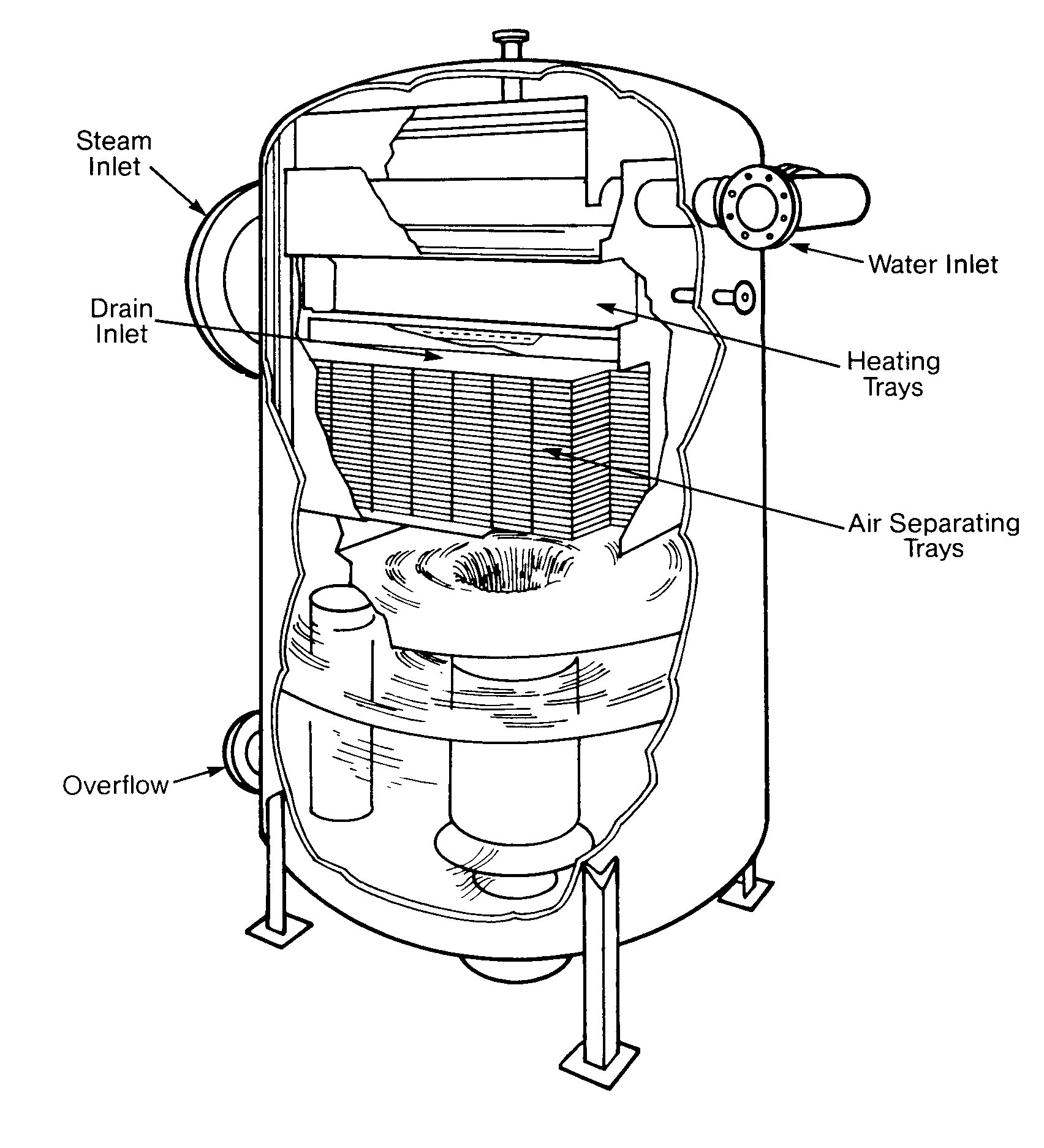
FIGURE 12.3
Tray-Type Deaerating Heater (Graver)
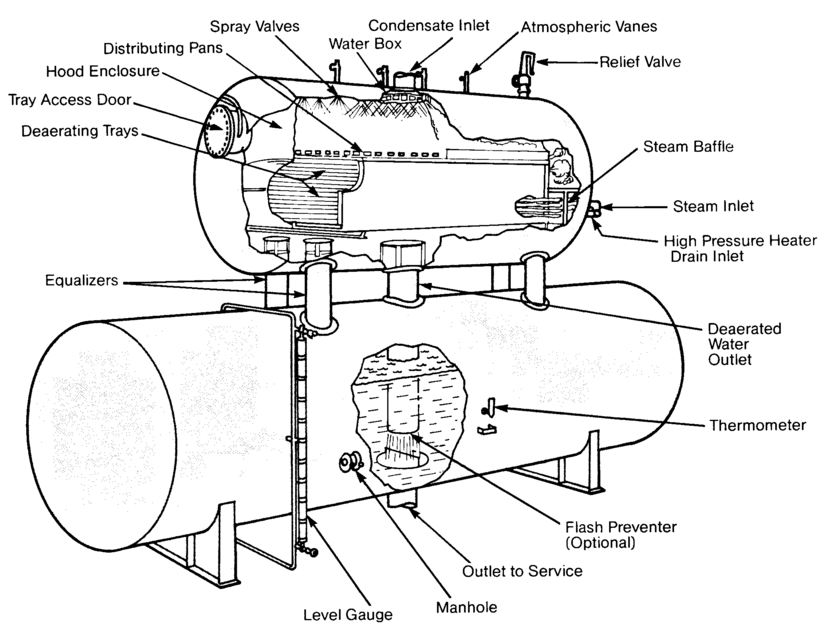
Spray-Type Deaerating Heaters
Spray-type deaerating heaters work on the same general philosophy as the tray-type, but differ in their operation. Spring-loaded nozzles located in the top of the unit spray the water into a steam atmosphere that heats it. Simply stated, the steam heats the water, and at the elevated temperature the solubility of oxygen is extremely low and most of the dissolved gases are removed from the system by venting. The spray will reduce the dissolved oxygen content to 20-50 ppb, while the scrubber or trays further reduce the oxygen content to approximately 7 ppb or less.
During normal operation, the vent valve must be open to maintain a continuous plume of vented vapors and steam at least 18 inches long. If this valve is throttled too much, air and nonconclensable gases will accumulate in the deaerator. This is known as air blanketing and can be remedied by increasing the vent rate.
For optimum oxygen removal, the water in the storage section must be heated to within 5 º F of the temperature of the steam at saturation conditions. From inlet to outlet, the water is deaerated in less than 10 seconds.
Storage Section
The storage section is usually designed to hold enough water for 10 minutes of boiler operation at full load.
Limitations
Inlet water should be virtually free of suspended solids that could clog spray valves and ports of the inlet distributor and the deaerator trays. In addition, spray valves, ports and deaerator trays may become plugged with scale that forms when the water being deaerated has high hardness and alkalinity levels. In this case, routine cleaning and inspection of the deaerator is very important.
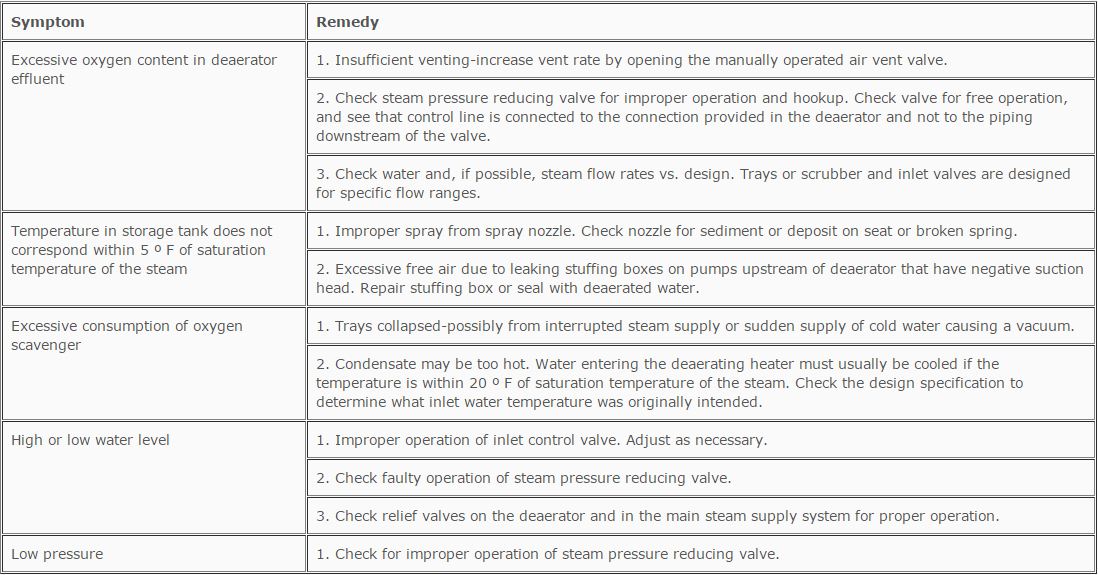
TABLE 12.2
Deaerating Heater Troubleshooting Guide
Rule of Thumb
The temperature of the bulk water in the storage section should be at least 2 º F for each I psig of the steam supply, up to 30 psig.
Example
5 psig steam
2ºF x 5 = 10ºF
Mimimum bulk water temperature
10 psig steam
2ºF x 10 = 20ºF
Minimum bulk water temperature
Saturation temperature = 227 ºF
212ºF + 10ºF = 222ºF
222 ºF
Saturation temperature = 240ºF
212 ºF + 20 ºF = 232 ºF
232 ºF
Economizers
Where economizers are installed, good deaerating heater operation is essential. Because oxygen pitting is the most common cause of economizer tube failure, this vital part of the boiler must be protected with an oxygen scavenger, usually catalyzed sodium sulfite. In order to insure complete corrosion protection of the economizer, it is common practice to maintain a sulfite residual of 5-10 ppm in the feed water and, if necessary, feed sufficient caustic soda or neutralizing amine to increase the feed water pH to between 8.0 and 9.0.
Below 900 psi excess sulfite (up to 200 ppm) in the boiler will not be harmful. To maintain blowdown rates, the conductivity can then be raised to compensate for the extra solids due to the presence of the higher level of sulfite in the boiler water. This added consideration (in protecting the economizer) is aimed at preventing a pitting failure. Make the application of an oxygen scavenger, such as catalyzed sulfite, a standard recommendation in all of your boiler treatment programs.
Treatment
The foregoing discussion shows the importance of proper deaeration of boiler feed water in order to prevent oxygen corrosion. Complete oxygen removal cannot be attained by mechanical deaeration alone. Equipment manufacturers state that a properly operated deaerating heater can mechanically reduce the dissolved oxygen concentrations in the feed water to 0.005 cc per liter (7 ppb) and 0 free carbon dioxide. Typically, plant oxygen levels vary from 3 to 50 ppb. Traces of dissolved oxygen remaining in the feed water can then be chemically removed with the oxygen scavenger.
Measurement of Dissolved Oxygen
Indigo Carmine- A colorimetric procedure for determining dissolved oxygen in the 0 to 100 ppb range. Standards are also available for high range (0-1 ppm),
Ampulmetric- This test offers ease of operation and minimum time in collecting reliable data. Capsules are available in the 0-100 ppb and 0-1 ppm range.
Oxygen Analyzers- Offers accurate reliable direct measurement in liquid streams. Used to monitor dissolved oxygen continuously or intermittently at various points in the condensate and feedwater systems.
Feed Water Quality
The major gaseous corrosive contaminants are removed through proper chemical and mechanical dearation. Migratory iron, migratory copper and other contaminants such as calcium, magnesium and silica must be conditioned within the boiler itself. Excursions of calcium, magnesium and silica can create deposition problems within the feed water train. Minimization of these contaminants prior to the feed water train is the most successful way of dealing with this problem.
Heat Exchange on Feed Water Train
Whenever heat can be recovered from another source, feed water is one of the best streams to receive this heat. The higher the temperature of the feed water going to the boiler, the more efficiently the boiler operates. However, any type of migratory deposition can impede the heat exchange process. Consequently, the highest quality feed water provides the highest heat exchange rate in either economizers or heaters. It is important to understand that none of these heat exchangers can be blown down during boiler operation.
Conclusions
The preparation of feed water for boiler injection has been optimized through the removal of undesirable salts and contaminants. In addition, the removal of oxygen and other non-condensable gases has been completed through chemical and mechanical deaeration. Establishing consistent feed water quality is extremely important in designing the internal boiler water treatment program.
Steam Generation Equipment, Potential Problems and Solutions
Discussion of Problems
Waterside Deposition
Boiler deposits result from hardness salts, metallic oxides, silica and a number of other feed water contaminants that can enter the system. In industrial boilers, it is cost prohibitive to eliminate all forms of contaminants in a pretreatment system. A controlled amount of contamination passes into the boiler with the feed water. Minimizing the adverse impact of these contaminants is the role of the boiler water treatment program.
Even the best controlled systems occasionally have upsets that cause excessive amounts of contamination to pass into the boiler. Some examples would be:
1. Carryover from a softener
2. Excess leakage from an ion exchange system
3. Contamination from leakage into condensate systems
4. Inadequate steam condensate protection programs resulting in high levels of corrosion products returning to the boiler
An internal boiler water treatment program must be forgiving enough to handle not only normal operating conditions but periodic upsets as well.
Mechanism of Deposition
In any steaming boiler, three basic conditions exist:
1. A circulation pattern is developed in the boiler due to steam bubbles, which alter the density of the boiler water. The hottest area of the boiler (where nucleate boiling occurs) is where the steam-boiler water mixture is the least dense. A rolling circulation pattern is developed as the steam proceeds through the boiler to its outlet for further plant use.
2. Based on convective, conductive and radiant heat transfer, thermal gradients are experienced throughout the steaming boiler. The hottest areas become primary deposition points due to high heat flux.
3. Flow patterns, velocities and concentrations of contaminants also follow the laws of gravity. Thus, any area of the boiler considered to be low flow may exhibit significant deposition.
In conclusion, the steaming boiler is a natural vessel for creating deposition of either precipitated soluble salts or migratory particulate contaminants. The ensuing discussions deal with both types of deposition.
Precipitated Soluble Salts. In the early years of boiler treatment, calcium sulfate was the principal scale product. This type of scale formation and deposition has been greatly minimized or eliminated. However, a good understanding of scale-forming tendencies and the resultant problems is necessary. The principal scaling and fouling ions are calcium, magnesium, iron and bicarbonate and carbonate alkalinity. Silica is also a potential foulant.
Scale formation is a function of two criteria:
1. The concentration and solubility limits of the dissolved salt
2. The retrograde solubility (inversely proportional to temperature) characteristic of some salts
In a steaming boiler, both of these conditions are met. While the boiler water is raised to a high temperature, the concentration of the dissolved salts is also increased. As steam is produced, dissolved salts remain in the boiler and continue to concentrate. Some salts may be soluble in the bulk boiler water. However, the boiler water immediately at the tube surface is considerably hotter than the bulk boiler water. As steam bubbles form near the tube wall, the soluble salts remain with the boiler water. This creates a localized high concentration of salts, even though the bulk boiler water may be well below saturation levels. The precipitation normally formed under these conditions has a crystalline structure and is relatively homogeneous.
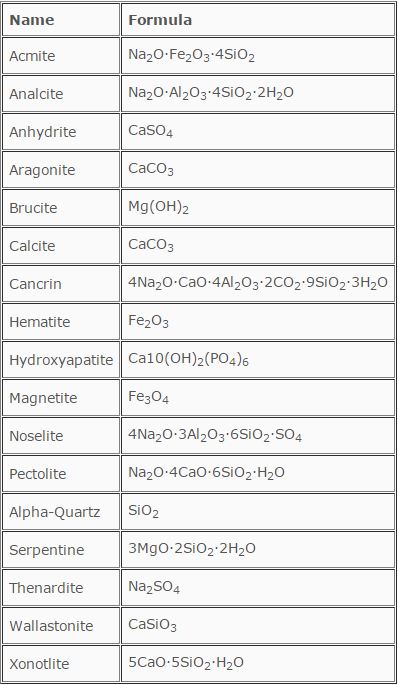
In actuality, the crystallization of salts is a relatively slow process. A well-defined crystal is formed and often results in a dense and highly insulating deposit. From a chemical equilibrium standpoint, reversibility of this reaction is quite low. Table 12.3 is a partial list of scaling and fouling deposits.
Particulant Migratory Contaminants. As boiler systems have become more efficient, higher quality feed water has been required. Consequently, the scaling salts have generally been removed prior to injection into the boiler. As a result, the particulate contaminants such as iron and copper become greater problems. It is not unusual to see a well-treated sodium zeolite boiler system with a coating of red iron oxide. This is typically a function of significant amounts of corrosion products returning from the condensate system and depositing in the boiler. For example, if iron is in a soluble form and not a particulate form, it can produce precipitates such as iron phosphates and iron silicates, which are extremely adherent deposits. In either case, the resultant precipitate or particulate has a strong affinity for tube metal through surface attraction. Water flow and circulation in tube banks will provide a force to keep the particles in suspension and moving. However, opposing this force are the surface attractions which are a function of the concentration of the suspended particles, the temperature and the boiler water chemistry.
TABLE 12.3
A Partial List of Boiler Deposits
As deposition begins, the localized temperature of the tube metal and deposit begins to increase. This temperature increase accelerates the deposition process. Depending on the type of particulate, a nonscale commonly known as baked-on sludge can form. This can be very hard. It forms dense deposits, particularly in high heat transfer areas.
In the high heat transfer areas, iron oxide can lose its water of hydration and become surface charged. The deposition of this iron is greatly enhanced by high tube wall temperatures. This type of deposition normally takes place in the furnace and water wall tubes, which provide for the highest amount of heat absorption in the boiler. Generally, this represents less than 10% of the surface area but approximately 50% of the heat absorption.
Conclusions
Regardless of the type of deposition, two very serious problems can occur:
1. Unscheduled shutdown due to tube failures from excessive deposition
2. Severe energy losses due to deposits retarding heat transfer in the critical areas of the boiler
Each of the above can be controlled through a sensible and well-applied internal boiler water program.
Boiler Waterside Corrosion
The most significant contributors to boiler waterside corrosion are dissolved oxygen, acid or caustic in the water and a high temperature. If any of these are uncontrolled, severe pitting, gouging and embrittling of the tube metal can occur, which will ultimately lead to failure. A good understanding of the mechanisms and control of these factors is extremely important.
Why Some Corrosion in the Boiler is Necessary
Water will rapidly corrode mild steel; as the temperature increases, the reaction accelerates. The following reaction is typical of iron corrosion in a boiler:
3 Fe + 4H20 » Fe3O4 + 4H2
Iron + Water/Steam » Magnetite + Hydrogen gas
The magnetite produced is black iron oxide. Under normal operating conditions, this is the typical product of corrosion. However, it is also this reaction that inhibits excessive corrosion in steaming boilers. In a new or clean boiler, the initial corrosion process produces this magnetite film as a tenacious layer at the steel surface. This magnetite layer prevents any further contact with the steel or water surface. Consequently, the corrosion reaction is self-inhibiting.
This magnetite layer grows to an approximate thickness of 0.0004-0.001 inches, at which point any further corrosion process ceases. Periodic weakening or damaging of this protective shell does occur, and proper internal boiler water treatment can repair this layer. The normal corrosion in a clean boiler system progresses at approximately 1 mm per year. The appropriate pH levels for maintenance of the magnetite layer is approximately 8.5-12.7, with most systems operating at a pH level of 10.5-11.5.
Other Causes of Corrosion
Dissolved Oxygen. In previous discussions concerning mechanical and chemical deaeration, removal of dissolved oxygen was considered essential. When dissolved oxygen enters the steaming boiler, corrosion manifests itself in the form of severe deep pits, almost exclusively at the water level in the steam drum. If oxygen attack has occurred, it is readily identifiable during inspection.
pH Variation (Acidic or Caustic Attack). Previously, a pH of 10.5-11.5 was identified as ideal for boiler operation, excluding high purity systems that could function on other types of treatment programs. Variations from the levels that are considered optimum for maintenance of the magnetite layer can cause general corrosion. Each of these will be discussed below.
Acidic Attack. If boiler water pH has dropped significantly below 8.5, a phenomenon called waterside thinning can occur. The normal manifestation of acidic attack is etching. In areas of higher flow, the surfaces are smooth. In addition, any stressed area would be a principal area for attack.
Caustic Attack. Caustic attack or, as it is more commonly known, caustic corrosion, is often encountered in phosphate treated boilers in which deposits occur in high heat transfer areas. In particular, boiler water can permeate the porous deposit. When it is coupled with significant heat flux, concentration of the boiler water occurs. Caustic soda (NaOH) is the only normal boiler water constituent that has high solubility and does not crystallize under these circumstances. This caustic concentration can be as high as 10,000-100,000 ppm. Localized attack due to the extremely high pH (12.9 +) will occur, as will the formation of caustic-ferritic compounds through the dissolving of the protective magnetite film. Once the process begins, the iron in contact with the boiler water will attempt to restore the protective magnetite film. Caustic corrosion (typically in the form of gouging) continues until the deposit is removed or the caustic concentration is reduced to normal.
Caustic attack typically appears in the form of irregular patterns and gouges. Frequently, the white salts associated with caustic attack remain in the tube samples. In addition, if caustic attack has proceeded for any extended period of time, significant levels of magnetic iron oxide can be found in any low flow area, such as a mud drum. This is essentially "stripping" of the magnetite film.
Steamside Tracking. This form of corrosive attack generally occurs in lower temperature areas of the boiler. A series of factors may permit stratified flow of steam and water in a given tube. When stratification occurs, the velocity of the steam-water mixture is not sufficient to maintain turbulent flow as the steam-water mixtures passes through the tubes. The affected tubes normally reveal gouging at the steam-water line and thinning under any deposits that have accumulated. Usually these systems contain a certain amount of caustic, which aggravates the rate of attack.
The phenomenon does occur in higher heat transfer areas as a function of a direct action between steam and hot steel. Metal temperatures of 900 º F (482 ºC) are required for this type of attack to proceed. Similar results in the form of thinning and gouging will be in evidence; however, a metallurgical examination might be required to determine the true mechanism of attack.
Steamside tracking or blanketing is a direct corrosive attack, similar to the acid or caustic attack. The other normally encountered form of corrosion is stress-related corrosion.
Stress Attack. Metallurgical examinations are required to identify the causes of stress attack. On occasion, intergranular or transgranular attack can be seen on tube specimens. The intergranular or transgranular attack can be a function of system condition or boiler water chemistry. It generally occurs in higher pressure systems.
Conclusions
Proper corrosion control is a function of several factors.
Proper boiler chemistry for operating pressures and conditions.
Close scrutiny and cntrol of boiler water chemistry.
Frequent testing of boiler water chemistry.
Thorough inspection of all waterside areas during shutdown.
Embrittlement
Embrittlement of boiler metal is normally referred to as caustic embrittlement or intercrystalline cracking. Failure of a boiler due to caustic embrittlement is normally undetectable during operating conditions; it generally occurs suddenly, with catastrophic results.
Three major factors must be present to cause intercrystalline cracking in boiler metal:
1. Leakage of boiler water must occur so as to permit the escape of steam and subsequent concentration of boiler water.
2. Attack of the boiler metal by concentrated caustic soda occurs from the concentrated boiler water.
3. There is high metal stress in the area of caustic concentration and leakage. In the past, caustic embrittlement failures have normally been associated with riveted seams in boiler drums. Modern welding techniques have eliminated this particular factor in today's boilers.
The actual phenomenon of caustic embrittlement is through high caustic concentrations traversing the grain boundaries within the crystalline structure of the metal. The caustic does not attack the crystals themselves, but rather travels between the crystals.
Corrective Approaches
It is an accepted fact that little control can be exerted over the factors of leakage and stress. Therefore, testing of the boiler water should be done to determine if embrittling characteristics exist. In the event the boiler water is found to be nonembrittling, leakage and stress can be ignored as far as embrittlement is concerned.
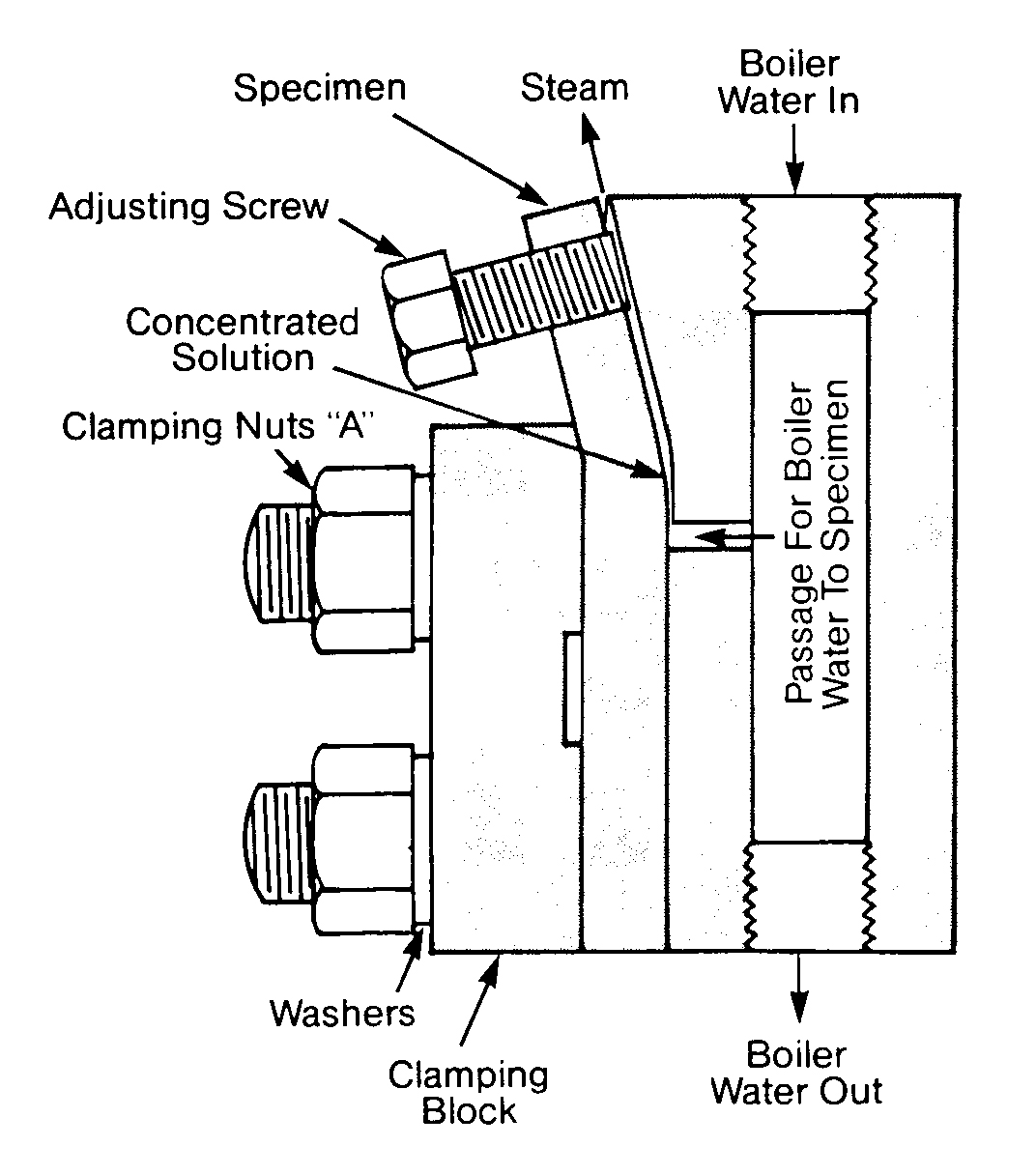
Embrittlement Detector
An embrittlement detector was patented by the United States Bureau of Mines and is covered by U. S. patents 2,283954 and 2,283955. Figure 12.4 shows a typical embrittlement detector. The normal installation area for an embrittlement detector is in the continuous blowdown line, providing the blowdown is approximately at boiler water temperature. Tests of 30, 60 or 90 days are run with the embrittlement detector, and the test bar is then submitted to bending tests.
FIGURE 12.4
Embrittlement Detector
If water is found to be embrittling, action can be taken to protect the boiler by adjusting boiler chemistry. In low pressure systems (less than 900 psi), sodium nitrate can be applied to inhibit embrittling tendencies. The formula for calculating the proper sodium nitrate/sodium hydroxide ratio in boiler water is as follows:
NaNO3/NaOH ratio = (ppm Nitrate as NO3) x 2.14
(ppm M.O. Alkalinity as CaCO3) - (ppm Phosphate as PO4)
The ratio is recommended by the U.S. Bureau of Mines and depends on boiler operating pressure, as foilows:
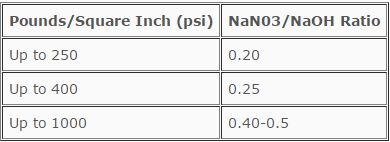
At boiler pressures in excess of 900 psi, boiler water chemistry can be adjusted to phosphate-pH control systems. The balance of pH and phosphate and the elimination of free caustic in the boiler water eliminates embrittling characteristics.
Summary
Boiler plants should have embrittlement tests run to determine the characteristics of the boiler water. If they have not been done, they should be recommended. If the testing has been accomplished and embrittling characteristics were not found. a statement should be made to this effect in any proposal for boiler water systems.
If the boiler water has been found to be embrittling, then appropriate adjustments in internal treatment are required.
Carryover
Carryover is generally considered to be any contaminant that leaves a boiler steam drum with the steam. It can be in solid, liquid or vaporous form. With higher operating pressures, higher superheat temperatures and the need for pure steam in certain processes, greater emphasis is placed on controlling the factors that minimize carryover.
For the sake of clarity, certain definitions are required:
1. Steam Purity- The amount of solid, liquid or vaporous contamination in the steam. Steam purity is normaily reported as total solids in parts per billion (ppb).
2. Steam Ouality- The amount of moisture in the steam. It is the weight of dry steam in the mixture of steam and water droplets. It is reported as a percentage.
Some of the effects of carryover are:
1. Deposition in regulators and valving
2. Deposition in superheaters
3. Deposition in control valves and turbines
4. Process contamination
Causes of Carryover
Simply put, carryover is a result of the incomplete separation of steam from the steam-water mixture in the boiler drum. There are a variety of factors that complicate separation. These are classified as chemical or mechanical factors.
Mechanical Factors. Mechanical causes vary by boiler design. the type of mechanical steam separating equipment, the fuels fired and the basic operational swing on the unit. In addition, steam load and boiler drum level have a significant effect on the amount of space available for steam separation from the steam-water mixture.
Chemical Factors. Foaming and selective vaporization are two basic mechanisms of chemical carryover. As the name implies, foaming is the formation of small bubbles on the surface of the boiler water. As these bubbles burst, the subsequent moisture is entrained with the steam. The levels of foam in the boiler steam drum also have a direct effect on carryover.
Selective vaporization occurs due to the solvent properties of steam for certain impurities that are normally present. All sodium-based salts found in boiler water are soluble in water and, to varying degrees, in the steam phase as well. Generally, the solubility of these salts is negligible below pressures of 2400 psig.
Selective vaporization of silica can occur at pressures as low as 400 psig. Usually, the problem of silica vaporization is not a concern at pressures below 900 psig.
Causes of Foaming
Generally, the causes of foaming are linked to the following:
1. High dissolved solids concentrations in the boiler water
2. High suspended solids concentrations
3. High alkalinity concentrations
Any of the above can affect chemical carryover. Other contaminants such as hydrocarbons can be saponified, and the resultant soaps can cause foaming. In addition, certain surface water supplies do contain synthetic detergents and wetting agents. Frequently, these synthetic detergents can combine with the hardness salts present to form insoluble precipitates. These precipitates tend to stabilize foam production in a boiler steam drum.
Correction of Carryover Problems
The ultimate use of steam in an industrial plant determines the degree of steam purity required for operation. While carryover can never be eliminated, even with the best boiler designs and boiler water chemistry, it can be reduced to a tolerable level. For example, if carryover levels can be reduced below 0.03 parts per million total solids, no problems would be anticipated with superheaters or turbines. (This is a general rule of thumb.)
Boiler manufacturers typically do not guarantee steam purity below 1.0 ppm total solids. However, modern boiler design routinely achieves levels of less than 0.1 ppm. A combination of good boiler design and good operation can work together to develop the steam purity levels required.
Mechanical Corrections
When a boiler must have optimum steam purity, operating pressure is a deciding factor in the design. At low pressures, the density of water is many times greater than that of steam. Therefore, adequate disengaging space and water surface area can be provided to achieve desired steam purity. As pressures increase, the density difference between steam and water decreases. To size a steam drum to separate steam and water adequately at higher pressure is cost prohibitive; therefore, mechanical separators are employed. The two major types of mechanical separation are primary separation and secondary separation (or steam scrubbing).
Steam washing is another form of improving steam purity, but this is generally limited to high pressure systems.
Primary Separation. This form of mechanical carryover prevention utilizes rapid and abrupt changes in the direction of steam flow. Major separation of the steam and water occurs in these primary devices. Simple baffles, curtain baffles, belly baffles, and cyclone separators are used in primary separation. Over 98% of the steam purity is achieved in the primary separation phase.
Secondary Separation. With the small amounts of moisture that remain after primary separation, the scrubbing or drying process has to be accomplished through rapid directional changes of the steam flow in conjunction with large surface areas for collection of the mist. In addition, steam velocity must be low to avoid reentrainment of the boiler water. Normally, screens or corrugated plates with close tolerances are used as steam scrubbers.
Chemical Corrections. Proper boiler water chemistry reduces the impact that dissolved salts, alkalinity, silica and hydrocarbon contaminants have on carryover tendencies. Table 12.44 shows the ABMA (American Boiler Manufacturers Association) standard boiler water concentrations for minimizing carryover. It should be recognized that these are guidelines only. Some plants can operate beyond these levels.
The best method of controlling chemically induced carryover is through restriction of all contaminants from the feed water. Sometimes, this is not possible. In addition, the carryover problem sometimes cannot be solved economically either through mechanical or chemical means. Effective use of boiler water anti-foam agents has significantly reduced carryover tendencies. Typically, these anti-foam materials are polyalcohols and amines. They function like other anti-foam agents. Where a chemical carryover problem exists, the results of anti-foam application can be dramatic. This frequently allows for good quality steam and higher concentrations in the boiler water chemistry.
TABLE 12.4
ABMA Standard Boiler Water Concentrations for Minimizing Carryover
* This value will limit the silica content of the steam to 0.25 ppm as a function of selective vaporization of silica. * Specific conductance is unneutralized.
Summary
The requirement for steam purity is a function of plant operation. If a problem exists, it is important to define the causes so the proper corrective action can be taken. Part of this problem definition must be accomplished through the measurement of steam purity (not covered in this section).
Heat Transfer Losses
In previous sections, discussion centered on deposition in high heat transfer areas. Much of this review centered on the potential for failure due to tube overheating or catastrophic corrosion. As the process of deposition continues, whether it is caused by poor external treatment or internal treatment, a slow and continuous loss of heat transfer and thermal efficiency occurs.
As a result, there are two significant reasons for maintaining the proper external and internal control: safe, reliable operation and efficient, cost-effective operation.
Thermal efficiency calculations can be made regarding the fireside and waterside of a steaming boiler. These are time consuming and they generally do not change dramatically over a short period of time. The ensuing sections discuss the approaches to internal boiler water treatment.
Internal Boiler Water Treatment
History
Internal boiler water treatment has come a long way in 75 years. Here is a chronology up to and including the late 1960s:
1. Pre-1920: Carbonate Cycle-This program introduced soda ash to prevent the formation of calcium sulfate scales. It replaced a rather difficult scale deposit (CaS04)with another scale that was somewhat easier to remove, calcium carbonate (CaC03).
2. 1920s: Phosphate Program-Soluble phosphate and alkali are added to the boiler system to promote the precipitation of calcium and magnesium ions in a desired sludge form. This sludge was removed via a manual blowdown, provided that the sludge remained in a fluid form.
A minimum pH value of 9.5 was required to precipitate the calcium properly. However, with the proper addition of alkalinity, pH levels of 10.5 plus were maintained to drive the reactions to completion. These reactions were also irreversible.
The desired boiler sludges are formed by the following reactions:
10Ca+2 + 6PO4-3 + 2OH- ± 3Ca3(PO4)2·Ca(OH)2
Calcium hydroxyapatite
3Mg-2 + 2SiO3-2 + 2OH- + H2O ± 2MgSiO3Mg(OH)2H2O
Serpentine
Both serpentine and caicium hydroxyapatite are relatively nonadherent to boiler metal and are easily removed by manual blowdown. The amount of phosphate, alkali and silicate required is based on these reactions plus an excess to drive the reactions to completion. Without enough hydroxyl alkalinity, the potential for calcium acid phosphate to precipitate is high. Calcium acid phosphate is a tenacious scale that cannot be removed while the boiler is operating.
3. 1950s: Phosphate-Organic-This program included the introduction of a surface-active material such as naturally occurring lignins, tanins, etc. These materials provided a dispersing and adsorptive action for maintaining the sludge in a fluidized form. This was an improvement over previous approaches.
4. 1960s: Phosphate-PoIymer-This provided a superior dispersive action to boiler water treatment. The synthetic polymers such as SSMA, PMA, and PAA were significantly more efficient than naturally occurring organic agents.
5. 1960s: CheIant Program-Coincidental with the development of synthetic polymers, chelant technology came into its own. In contrast to the precipitating type of programs identified with phosphate base systems. Chelation is a solublizing approach.
High pressure boiler water control systems have not been reviewed above. They are specialized and unique programs based on boiler design, operating pressure and feed water quality.
General Discussion
When choosing an internal treatment program, many factors must be considered. However, one point remains extremely important: Any boiler opening will be on outstanding event, if proper control and situational responses have been carried out in an effective monner The final choice should be a program with excellent control that plant operators are most comfortable with. In addition, it should be a cost-effective program. The following sections present the pros and cons of precipitating and solubilizing programs.
Precipitating Programs (Phosphate-Polymer).
This type of program causes sludge to form and therefore requires continuous blowdown. Deposition problems are possible in the higher heat transfer areas of the boiler. On the positive side, phosphate-polymer programs can essentially handle any feed water hardness and be cost effective. This is particularly true of the new synthetic polymers.
Generally, a phosphate-treated boiler leaves a light grey deposition of approximately 1 / 16 inch. The rolled tube ends and the steam drums frequently have larger amounts of deposition. Sludge is evident in the mud drum and this deposition is easily waterwashed.
Solubilizing Programs (Chelant-Polymer).
in contrast to precipitating programs, chelating agents form water-soluble complexes with calcium, magnesium. iron and other metal ions. On the pro side, no sludge is formed, better heat transfer is achieved and the program has some forgiveness. Disadvantages include its expense over precipitating programs with high feed water hardness. In addition, chelant testing can be difficult for inexperienced operators.
During a chelant control program inspection, a light iron oxide dusting would be noted. All tube services should be clean and the rolled tube ends should show no deposition. All baffle plates should be clean and only a small amount of debris should be found in the mud drum.
While some chelant corrosion did occur in the 1960s, this was due to improper application and inadequate testing. Significant overfeed occurred in each documented case of chelant corrosion. This is no longer a problem today.
Summary
Present boiler water technology can provide efficient, cost-effective and reliable operation for industrial boiler systems. Selection of the best internal treatment program is a function of many factors. However, the ability to control the program should be the first consideration. One of the significant methods of control is blowdown regulation, which is covered in the next section.
Blowdown Control
The main purpose of blowdown is to maintain the solids content of the boiler water within prescribed limits. This would be under normal steaming conditions. However, in the event contamination is introduced in the boiler, high continuous and manual blowdown rates are used to reduce the contamination as quickly as possible.
Table 12.4 indicates certain ABMA guidelines have been provided for safe and reliable operation. In actual situations, some of these guidelines have been exceeded quite successfully. Because each boiler and plant operation is different, maximum levels should be determined on an individual basis.
Bottom Blowdown
By definition, bottom blowdown is intermittent and designed to remove sludge from the areas of the boiler where it settles. The frequency of bottom blowdown is a function of experience and plant operation. Bottom blowdown can be accomplished manually or electronically using automatic blowdown controllers.
Continuous Blowdown
Frequently used in conjunction with manual blowdown, continuous blowdown constantly removes concentrated water from the boiler. By design, it is in the area of highest boiler water concentration. This point is determined by the design of the boiler and is generally the area of greatest steam release.
Continuous blowdown allows for excellent control over boiler water solids. In addition, it can remove significant levels of suspended solids. Another advantage is that the continuous blowdown can be passed through heat recovery equipment.
Summary
Proper boiler blowdown control in conjunction with proper internal boiler water treatment will provide the desired results for a boiler water program. Many modern devices can automate boiler blowdown, thereby increasing the overall efficiency of the unit.
Steam Condensing and Receiving Systems
Condensate system equipment is defined as condensers, receivers, regulators, traps and piping. Normally considered a potential maintenance nightmare, a properly treated and operated condensate system can be a moneymaker for an industrial plant.
Potential Corrosion Problems
The principal problems associated with return condensate systems deal with corrosion by oxygen, carbon dioxide and copper complexing agents. With significant corrosion, fouling of the return piping can also occur. Each of these sources of corrosive attack is discussed below.
Oxygen Corrosion. The corrosion of a condensate system by oxygen is in the form of severe pitting. As in the feed water train, oxygen corrosion is easily recognized by large pits produced at the point of attack.
Active oxygen pitting is easily recognized by the black oxide present in the pit. The surrounding area may be red ferric oxide, but this is a secondary product of the corrosion mechanism. If the pit itself contains red iron oxide, that simply indicates a past corrosion problem.
Oxygen has many opportunities to enter a condensate system. Leaks due to expansion and contraction of the return system are possible in receiving tanks, pumps, etc. Poor mechanical and chemical deaeration of the feed water can also introduce oxygen in the condensate system.
Carbon Dioxide Corrosion. Carbon dioxide can enter a condensate system as a dissolved gas or it can be chemically combined in the bicarbonate or carbonate alkalinity of the feed water. Generally. dissolved carbon dioxide is removed in the deaerating heater. The following reactions show the breakdown of naturally occurring bicarbonate and carbonate alkalinity to carbon dioxide.
Reaction 1:
NaHCO2 + heat ± NaOH + CO2
Reaction 2:
Na2CO3 + H2O + heat ± 2 NaOH + CO2
Reaction #1 proceeds to completion. Reaction #2 is only about 80% complete.
The manifestation of carbon dioxide corrosion is generalized loss of metal, typified by grooving of the pipe walls at the bottom of the pipe: attack occurs at the threaded or stressed areas. This is the most common form of condensate system attack.
Copper Complexing Corrosion.
The most commonly found copper complexing agent in condensate systems is ammonia. This can be present in low concentrations due to the decomposition of organic contaminants, hydrazine or amine type treatment chemicals. A good understanding of the levels of ammonia is necessary in the event the condensate system contains copper-bearing alloys.
Corrosion Control Programs
The basic approach to chemical treatment of condensate systems is through the use of neutralizing amines, filming amines, combinations of both amines and hydrazine.
Neutralizing Amines.
Simply stated, neutralizing amines hydrolyze in water to generate the necessary hydroxide ions required for neutralization of the carbon dioxide. The normal approach to treating systems with these amines is to feed sufficient quantity to neutralize the carbon dioxide and then provide small additional amounts to buffer the pH to 8.5 or 9.0. At this pH, continued preservation of the magnetite film is also achieved. It is also implied that corrosion will not exist at a pH>8.0-8.5.
A variety of amines are available for condensate neutralization and pH elevation. Based on the complexity of a condensate system, several methods are employed for determining which neutralizing amine is best for the application. Frequently, a blend of amines may appear to be optimum. One method of selection deals with the distribution ratio of the amine. This is defined as the amount of amine in the vapor phase divided by the amount of amine in the condensate. The distribution ratios for commonly utilized neutralized amines are shown in Table 12.5.
TABLE 12.5
Distribution Ratios for Neutralized Amines
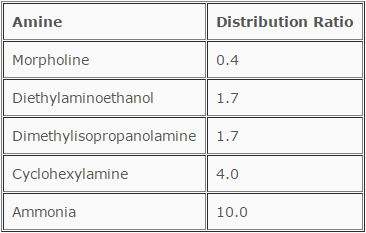
For example, because of its distribution ratio, morpholine is an excellent choice for plants that have operating turbines. It begins to leave the gaseous phase as the first condensation occurs in the wet end of the turbine. Conversely, cyclohexylamine will preferentially stay with the steam for a longer period of time. Blends of these amines or others are most desirable for plants with extensive condensate systems.
Filming Amines.
Filming amines function by forming a protective barrier against both oxygen and carbon dioxide attack. These amines form films directly with the condensate line metal and develop a barrier to prevent contact of the corrosive condensate with the return piping. By design, film formers have been developed to function best at a pH of 5.5-7.5. In addition, these amines are highly surface-active and will slough loosely adherent iron oxide and other corrosion products back to receiving points or to the boiler. Care must be exercised with the feed of filming amines.
Combination Amines. Over the past several years, combinations of filming and neutralizing amines have been shown to be extremely effective, particularly in complex systems. While the combination amine is still functionally a filmer, the neutralizing amine portions provide for reduction in fouling potential and more uniform coverage of the filmer.
Filming amines and combination amines are generally fed to steam headers. Dosages are based on steam production.
Control of oxygen attack can be accomplished through the proper selection of filming amine programs. In addition, hydrazine has been injected into condensate systems to protect against oxygen attack.
General Commentary
Failure Analysis
While some failure analysis was discussed earlier in this Chapter, the following is a summary of the typical types of boiler failures.
Metallurgical Failures and Analysis
Elevated Temperature Failure. Temperature failure can occur through rapid overheating or long-term overheating. Rapid overheating is characterized by thin-lipped ruptures and complete microstructural transformation due to overheating to above 1333 ºF . It is typically caused by starvation of a tube due to a blockage or rapid start-ups
Fatigue Failures. Fatigue cracking is caused by cyclic stresses. Typical causes are:
1. Vibration (failure usually occurs near an unyielding restraint)
2. Thermal expansion /contraction of boiler tubing (cracking can occur along entire tube length)
Metallographic examination of the cracking will reveal transcrystalline, unbranched cracks.
Caustic Embrittlement. Caustic embrittlement is a form of stress corrosion cracking that occurs in mild and low alloy steel due to the conjugant action of an enduring tensile stress and concentrated solution of sodium hydroxide.
The stress may be residual (from welding or prior cold work) or applied. Metallographic examination shows continuous, branched, intergranular cracking.
Corrosion Failure. Corrosion failure occurs as pitting, thinning or gouging.
Pitting corrosion can occur due to the presence of free oxygen entering the boiler with the feed water as a result of incomplete deaeration and/or chemical scavenging. Oxygen pitting can also occur during downtime due to improper storage procedures. Plating of copper metal onto a boiler tube side during acid-cleaning can also result in pitting due to dissimilar metal corrosion.
General corrosion or thinning can occur due to:
1. Acid attack
2. Chelant attack
3. Steam blanketing (localized to the hot side of the tubing)
Caustic gouging typically occurs under deposits due to the concentration of sodium hydroxide via evaporation of the boiler water. Caustic attack can also occur in conjunction with steam blanketing.
Optimum Approach to Boiler Water Treatment
There are many ways to approach the selection of a sound and effective internal boiler water treatment program. Above all, the best selection can be made with a proper understanding of system design, capabilities, water chemistry and common sense.